- EN - English
- CN - 中文
Generation of a Human Conditionally Immortalized Cell-based Multicellular Spheroidal Blood-Brain Barrier Model for Permeability Evaluation of Macromolecules
建立用于大分子渗透性评价的人条件永生细胞多细胞球形血脑屏障模型
发布: 2022年08月05日第12卷第15期 DOI: 10.21769/BioProtoc.4465 浏览次数: 3630
评审: Pilar Villacampa AlcubierreAchira RoyXuecai Ge
Abstract
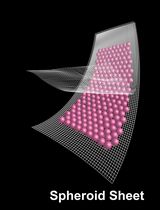
There is an urgent need for the development of brain drug delivery carriers based on middle-sized or macromolecules, to which in vitro blood-brain barrier (BBB) models are expected to contribute significantly through evaluation of BBB permeability. As part of efforts to develop such models, we have been working on human conditionally immortalized cell-based multicellular spheroidal BBB models (hiMCS-BBB models), and we herein introduce the model development protocol. Briefly, astrocytes are first seeded in an ultra-low attachment 3D cell culture plate, to make the central core (Day 0). Next, pericytes are added over the core, to form an outer layer (Day 1). Then, brain microvascular endothelial cells are further added to each well, to create the outmost monolayer serving as the BBB (Day 2). Finally, the spheroids cultured for two days (on Day 4) can be used for assays of interest (e.g., antibody permeability assays). Neither special equipment nor techniques are required to produce hiMCS-BBB models. Therefore, the protocol presented here will not only facilitate the model sharing among the BBB community but also provide some technical clues contributing to the development of similar MCS-BBB models using other cell sources, such as primary or iPS-derived BBB cells.
Graphical abstract:

Background
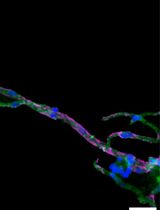
The blood-brain barrier (BBB) forms a nearly impregnable wall preventing the distribution of numerous blood-borne middle-sized and macromolecules (middle/macromolecules) and low-molecular-weight drugs into the human brain (Sanchez-Cano et al., 2021; Segarra et al., 2021). As such, it often imposes a frustrating barrier to brain drug delivery efforts, thus rendering central nervous system (CNS) diseases among the most difficult to treat pharmaceutically. Brain microvascular endothelial cells (BMECs), which work with the support of pericytes and astrocytes, make up the critical components of the BBB. The pericytes are located in the immediate vicinity of the BMECs, while the astrocytes wrap them through their endfeet. This structural integrity is essential for BBB functions (Ballabh et al., 2004; Abbott et al., 2006; Daneman et al., 2015). The two primary functional characteristics of the BBB are tight/adherens junctions and efflux transporters. The former tightly seals BMEC plasma membranes to limit paracellular transport of various substances, including middle/macromolecules, while the latter are localized on the vascular side of BMECs and actively pump out their substrates into circulation (Ballabh et al., 2004; Abbott et al., 2010). Therefore, as part of efforts to create effective therapeutic approaches to CNS diseases, it is necessary to develop BBB-permeable drug delivery system (DDS) carriers that can overcome this barrier.
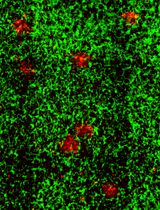
From that point of view, receptor-mediated transcytosis (RMT) occurring at the BBB has gained significant attention (Lajoie et al., 2015; Fang et al., 2017). Physiologically, after binding to their middle/macromolecule ligands at the blood side of BMECs, RMT receptors undergo endosomal vesicle formation to travel to the brain side, where they eventually release their cargo into the brain via exocytosis (Freskgård et al., 2017; Kouhi et al., 2021; Zhou et al., 2021). One intriguing idea for facilitating the development of brain DDS carriers involves taking advantage of those RMT processes, as exemplified by antibodies targeting transferrin or insulin receptors expressed in BMECs (Boado et al., 2016; Sonoda et al., 2018), resulting in an ongoing urgent need for effective and high-throughput evaluations of their BBB permeabilities. To this end, there have been a variety of next-generation in vitro BBB models based on microphysiological systems (MPSs), including BBB-on-a-chip, organoids, and spheroids (Cho et al., 2017; Wevers et al., 2018; Simonneau et al., 2021). These models feature high functionality derived based on their reproduction of the structure and surrounding microenvironment of the BBB in vivo (specifically, MPSs).
As part of efforts to facilitate the development of such models, we have been working on human conditionally immortalized cell-based multicellular spheroidal BBB models (hiMCS-BBB models) consisting of human conditionally immortalized BMECs (HBMEC/ci18 cells) (Kamiichi et al., 2012; Ito et al., 2019), astrocytes (HASTR/ci35 cells) (Furihata et al., 2016; Kitamura et al., 2018), and brain pericytes (HBPC/ci37 cells) (Umehara et al., 2018). These cells carry immortalization genes that enable them to extensively proliferate, while their status can be changed simply by varying the cell culture temperature (33 °C for growth and 37 °C for differentiation — for details, please refer to our above-cited previous papers).
In hiMCS-BBB models, HASTR/ci35 cells shape the central core, which is covered by an HBPC/ci37 cell layer. That layer is further surrounded by an HBMEC/ci18 cell monolayer serving as the BBB (Kitamura et al., 2021, 2022). This structural feature allows us to perform several BBB functional analyses, such as transporter activity assessments, and immune cell recruitments (Kitamura et al., 2021, 2022). In particular, the models exhibit RMT functions that are sufficiently high for use in evaluating the BBB permeability of antibodies and peptides (Kitamura et al., 2022). Additionally, the immortalized cells used are both scalable and easy to handle, and neither special equipment nor techniques are required to produce the models (as described herein). Owing to all these advantages, hiMCS-BBB models are expected to have great potential for use in developing novel middle/macromolecule-based DDS carriers.
In this paper, we describe the method used for constructing hiMCS-BBB models together with the culture methods for the human immortalized BBB cells. In alignment with that, we will also introduce an example of a middle/macromolecule permeability assay performed using hiMCS-BBB models. Through this publication, we aim not only to share hiMCS-BBB models among the BBB community but also to provide some technical clues that may contribute to the development of MCS-BBB models using other cell sources, such as primary or iPS-derived BBB cells, although some modifications may be necessary.
Materials and Reagents
Note: Most products can be replaced by those from other suppliers, but it is uncertain whether this is also true for the marked products (*) since we have not tested other materials.
Low binding 200 µL tips (TreffLab, catalog number: 96.11179.4.01)
Normal tips (Nacalai Tesque, catalog numbers: 19166-54 [10 µL], 19167-44 [200 µL], 19168-34 [1,000 µL])
Violamo Disposable Pipette II (VIOLAMO, catalog numbers: 2-5237-12 [2 mL], 2-5237-03 [5 mL], 2-5237-04 [10 mL], 2-5237-05 [25 mL])
Cryotubes (Thermo Fisher Scientific, catalog number: 363401PK)
15-mL centrifuge tubes (Thermo Fisher Scientific, catalog number: 339650)
50-mL centrifuge tubes (Thermo Fisher Scientific, catalog number: 339652)
1.5 mL tubes (WATSON, catalog number: 131-715C)
Protein LoBind 1.5 mL tubes (Eppendorf, catalog number: 22431081)
Collagen I-coated 60-mm dishes (IWAKI, catalog number: 4010-010)
Collagen I-coated 100-mm dishes (IWAKI, catalog number: 4020-010)
Note: Other collagen type I-coated products may be used.
PrimeSurface 96V plates (96-well V-bottom plates)* (Sumitomo Bakelite, catalog number: MS-9096V)
Cell counting slides (Logos Biosystems, catalog number: L12001)
Slide glass 76 × 26 mm (slide glass) (Matsunami-glass, catalog number: MAS-01)
Cover glass 24 × 60 mm (cover glass) (Matsunami-glass, catalog number: C024601)
100-mL flask (IWAKI, catalog number: 4980FK100)
Parafilm
Nail polish
Human astrocytes/conditionally immortalized clone 35 (HASTR/ci35 cells)* (Furihata et al., 2016; Kitamura et al., 2018)
Human brain pericytes/conditionally immortalized clone 37 (HBPC/ci37 cells)* (Umehara et al., 2018)
Human brain microvascular endothelial cells/conditionally immortalized clone 18 (HBMEC/ci18 cells)* (Kamiichi et al., 2012; Ito et al., 2019)
Blasticidin S (InvivoGen, catalog number: ant-bl-1)
Astrocyte Medium kit (consisting of the basal medium and culture supplements)* (Thermo Fisher Scientific, catalog number: A1261301)
Pericyte Medium kit (consisting of the basal medium and culture supplements)* (ScienCell, catalog number: 1201)
VascuLife VEGF Comp kit (consisting of VascuLife BM and culture supplements) (Lifeline Cell Technology, catalog number: LEC-LL0003)
Note: EBM-2 (Lonza, catalog number: CC-3162) can be used instead.
Penicillin-streptomycin (Nacalai Tesque, catalog number: 26253-84)
Bambanker (NIPPON Genetics, catalog number: CS-02-001)
Dulbecco’s phosphate buffered saline Ca, Mg free (D-PBS(-)) (Nacalai Tesque, catalog number: 14249-24)
D-PBS(+) preparation reagent (Ca, Mg solution) (Nacalai Tesque, catalog number: 02492-94)
Hanks’ balanced salt solution (HBSS(+)) (Nacalai Tesque, catalog number: 09735-75)
2.5 g/L-trypsin/1 mmol/L-EDTA (trypsin) (Nacalai Tesque, catalog number: 32777-15)
Trypan blue (Wako, catalog number: 207-17081)
4% paraformaldehyde (PFA) (Nacalai Tesque, catalog number: 09154-85)
Fluoro-KEEPER Antifade Reagent, Non-Hardening Type (antifade reagent) (Nacalai Tesque, catalog number: 12593-64)
Methyl cellulose-viscosity: 4,000 cP (methyl cellulose)* (Sigma-Aldrich, catalog number: M0512-100G)
MEM189 [Alexa Fluor 647] (Novus Biologicals, catalog number: NB500-493AF647)
CellTracker Orange CMTMR Dye (Invitrogen, catalog number: C2927)
Red-fluorescent Cytoplasmic Membrane Staining Kit (PromoKine, catalog number: PK-CA707-30023)
CellTracker Green CMFDA Dye (Invitrogen, catalog number: C2925)
AM (astrocyte medium) (see Recipes)
PM (pericyte medium) (see Recipes)
VascuLife medium (see Recipes)
Spheroid medium (see Recipes)
D-PBS(+) (see Recipes)
Equipment
Clean bench (or safety cabinet)
Water bath set at 37 °C
Water bath set at 60 °C
Centrifuge (e.g., Kokusan, catalog number: H-19Rα)
Aspirator
Phase contrast microscope (e.g., Nikon, ECLIPSE Ts2)
Confocal laser scanning microscope (e.g., OLYMPUS, FLUOVIEW FV3000)
Humidified incubator set at 33 °C with 5% CO2/95% air
Humidified incubator set at 37 °C with 5% CO2/95% air
Automated cell counter (e.g., Logos Biosystems, catalog number: L10001SS)
Fridge (4 °C) and freezers (-20 °C and -80 °C)
Liquid nitrogen storage
Electric pipettor (e.g., Greiner Bio-One, catalog number: J847050)
Pipettes (e.g., HTL LAB SOLUTIONS, catalog number: 7904)
Stirring bar (e.g., AS ONE Corporation, catalog number: 1-4206-27)
Magnetic stirrer
Autoclave (e.g., HIRAYAMA, HV-2LB)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Isogai, R., Morio, H., Okamoto, A., Kitamura, K. and Furihata, T. (2022). Generation of a Human Conditionally Immortalized Cell-based Multicellular Spheroidal Blood-Brain Barrier Model for Permeability Evaluation of Macromolecules. Bio-protocol 12(15): e4465. DOI: 10.21769/BioProtoc.4465.
分类
药物发现 > 药物筛选
神经科学 > 神经系统疾病 > 血脑屏障
细胞生物学 > 细胞工程 > 组织工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link