- EN - English
- CN - 中文
Time-off-pick Assay to Measure Caenorhabditis elegans Motility
时间-间隔法测定秀丽隐杆线虫运动能力
发布: 2022年06月20日第12卷第12期 DOI: 10.21769/BioProtoc.4436 浏览次数: 3174
评审: Juan Facundo Rodriguez AyalaMatthias RieckherRajesh RanjanAnonymous reviewer(s)
Abstract
Caenorhabditis elegans is a simple metazoan that is often used as a model organism to study various human ailments with impaired motility phenotypes, including protein conformational diseases. Numerous motility assays that measure neuro-muscular function have been employed using C. elegans. Here, we describe “time-off-pick" (TOP), a novel assay for assessing motility in C. elegans. TOP is conducted by sliding an eyebrow hair under the mid-section of the worm and counting the number of seconds it takes for the worm to crawl completely off. The time it takes for the worm to crawl off the eyebrow hair is proportional to the severity of its motility defect. Other readouts of motility include crawling or swimming phenotypes, and although widely established, have some limitations. For example, worms that are roller mutants are less suitable for crawling or swimming assays. We demonstrated that our novel TOP assay is sensitive to age-dependent changes in motility, thus, providing another more inclusive method to assess motor function in C. elegans.
Graphical abstract:

Conceptual overview of the “time-off-pick” (TOP) assay. Various C. elegans models exhibit age-dependent defects in motility. The time it takes for a worm to crawl off of an eyebrow pick that is slid under its mid-section is measured in TOP seconds. A greater TOP is indicative of a greater motility defect. Eventually, worms with phenotypes that lead to paralysis will not be able to leave the pick.
Background
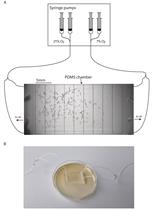
Caenorhabditis elegans is a 1-mm long nematode often used as a model organism for studying various human diseases or conditions that induce neuro-muscular defects. As such, motility assays are commonly used to assess motor impairments in C. elegans. Common motility assays measure parameters associated with swimming and crawling phenotypes, which have proven to be very successful and effective readouts (Cohen et al., 2012; Winter et al., 2016). However, the aforementioned assays can be time-consuming, may require specialized equipment, and are less compatible with certain C. elegans phenotypes, such as the roller phenotype, which is commonly used as a selection marker when generating transgenic C. elegans lines. The protocol presented in this paper, “time-off-pick” (TOP), describes a novel phenotypic assay for measuring motility in C. elegans. Our method is less elaborate, does not require specialized equipment, and is more inclusive to a variety of genetic backgrounds, including those that exhibit the roller phenotype. TOP involves sliding an eyebrow hair under the mid-section of the worm and counting the number of seconds it takes for the worm to crawl off (Video 1). The worm is not lifted by the eyebrow hair; rather, the hair remains stationary after being gently placed between the worm and the surface of the agar on nematode-growth media (NGM) plates, with care taken not to poke the side of the worm with the eyebrow hair. A longer TOP is indicative of a greater motility impairment. This method allows the experimenter to study C. elegans strains, mutations, conditions, or treatments that alter motility.
To demonstrate the feasibility of our method, we used C. elegans strains that are known to affect motility: lines carrying transgenic polyglutamine (polyQ) tracts (Morley et al., 2002; Brignull et al., 2006; Mohri-Shiomi and Garsin, 2008) and a mutant strain that manifests in a temperature-sensitive motility defect (Macleod et al., 1977). PolyQ tracts aggregate in a polyQ length- and an age-dependent manner, and increased polyQ aggregation is associated with increased motility defects (Morley et al., 2002; Brignull et al., 2006; Mohri-Shiomi and Garsin, 2008). In our previous work, we demonstrated that the results obtained from the TOP assay matched those from the swimming assay (“thrashing”) (Walker et al., 2021). Here, we show our TOP method can successfully assess changes in age- and polyQ length-dependent motility in worms harboring polyQ tracts in the intestine (Figure 1A), muscle (Figure 1B), and neurons (Figure 1C). To demonstrate the versatility of our method, we used a strain (CB1301) that expresses a temperature-sensitive mutation in myosin heavy chain gene, unc-54 (e1301), that results in impaired motility at restrictive temperatures (Macleod et al., 1977; Gidalevitz et al., 2006). We found that TOP detected a temperature-induced motility impairment 1 h after worms were placed at the restrictive temperature (Figure 1D). Together, these results demonstrate the use and efficacy of the TOP phenotype, which provides a validated and sensitive addition to existing motility assays.
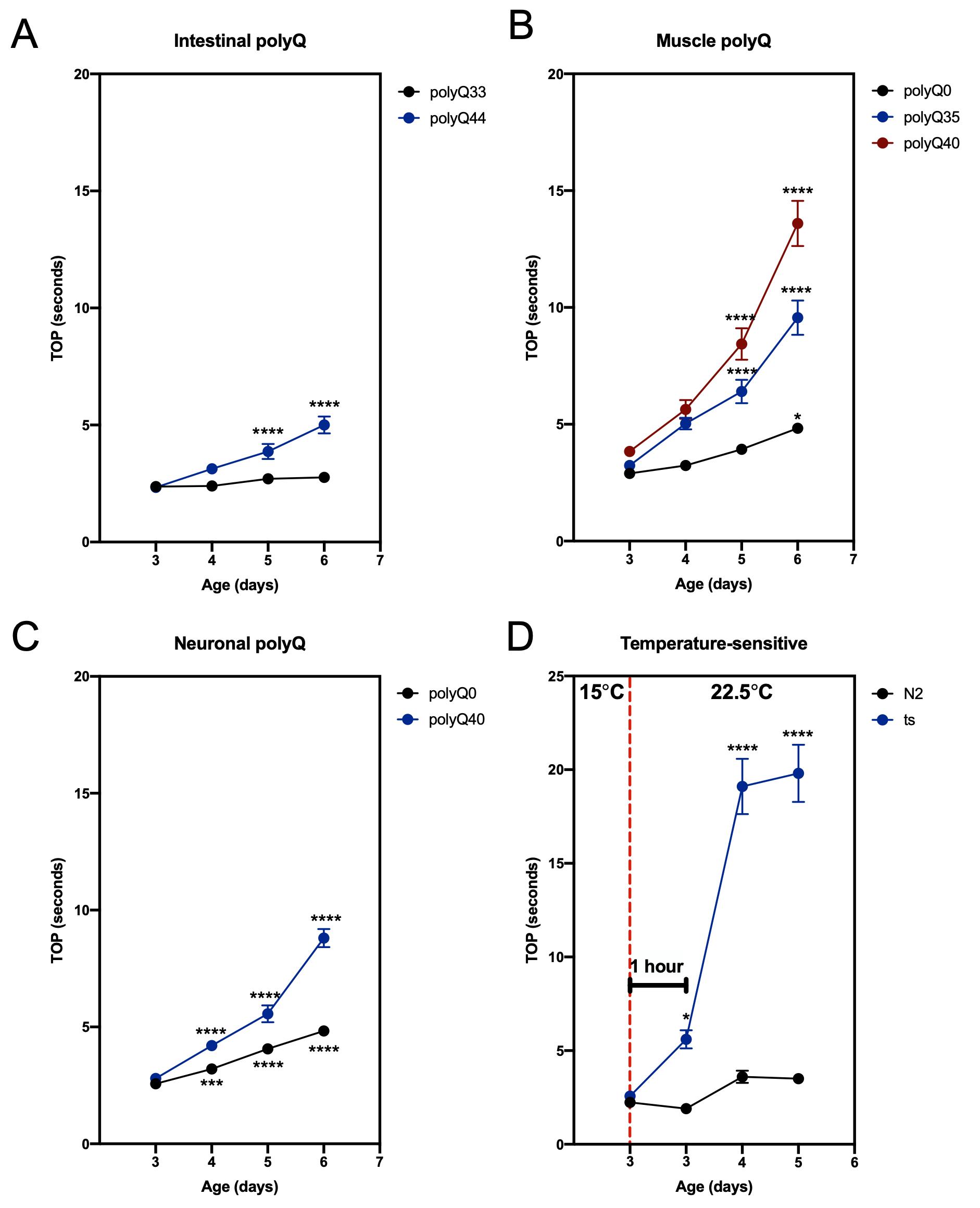
Figure 1. TOP measurement in C. elegans expressing polyQ and a temperature-sensitive mutation. (A–C) The TOP phenotype positively correlates with C. elegans age and polyQ length in the (A) intestine (rollers): polyQ44: AM738 rmIs297[vha-6p::q44::yfp; rol-6 (su1006)]; polyQ33: AM712 rmIs281[vha-6p::q33::yfp; rol-6 (su1006)], (B) muscle: polyQ40: AM141 rmIs133[unc-54p::q40::yfp]; polyQ35: AM140 rmIs132[unc-54p::q35::yfp]; polyQ0: AM134 rmIs126[unc-54p::q0::yfp], and (C) neurons: polyQ40: AM101 rmIs110[F25B3.3P::q40::yfp]; polyQ0: AM52 rmIs182[F25B3.3p::q0::yfp]. Worms were cultured and maintained at 22.5°C. (D) Motility of worms (CB1301) expressing a temperature-sensitive mutation (ts) in myosin heavy chain, unc-54(e1301). Nematodes cultured and maintained at 20°C harboring the mutation display a significant increase in TOP (seconds) after 1 h at a restrictive temperature of 22.5°C (data point immediately to the right of the red, dashed line), whereas wild-type worms (N2), exhibit no significant change. Motility impairment continues to worsen over the next 48 h at the restrictive temperature. Data are represented as the average TOP per worm. Each data point is an average of two independent experiments with a total of 30 worms. Error bars represent standard error of the mean (SEM). Statistical analysis was calculated using one-way analysis of variants (ANOVA) followed by multiple comparison Dunnett’s post-hoc test (*P < 0.05, ***P < 0.001, **** P < 0.0001).
Materials and Reagents
Note: Unless otherwise specified, all reagents are stored at room temperature.
Erlenmeyer flasks (2,000 mL and 1,000 mL)
6 cm Petri dishes (Genesee Scientific, catalog number: 32-105G)
Magnetic stir bar
Autoclave tape
Caenorhabditis elegans
500 mL Olympus vacuum filter flasks (Genesee Scientific, catalog number: 25-227)
Agar (Fisher Scientific, catalog number: BP1423)
Double distilled water (ddH2O)
NaCl (Fisher Scientific, catalog number: S671-500)
Trypticase-peptone (Gibco, catalog number: 211921)
Cholesterol (MP Biomedicals, catalog number: ICN10138201)
Ethanol, 200-proof (Decon Laboratories, catalog number: 04-355-223)
CaCl2·2H2O (Fisher Scientific, catalog number: C79-500)
MgSO4·7H2O (Fisher Scientific, catalog number: A14491)
KH2PO4 (Fisher Chemical, catalog number: P285-3)
NA2HPO4·7H2O (Fisher Scientific, catalog number: S373-500)
M9 Minimal Medium (see Recipes)
Cholesterol Stock Solution (see Recipes)
1 M CaCl2 Stock Solution (see Recipes)
1 M MgSO4 Stock Solution (see Recipes)
1 M KH2PO4, pH 6.0 Stock Solution (see Recipes)
Eyebrow hair pick (see Recipes)
NGM Plates (see Recipes)
Equipment
Zeiss Stemi 305 stereo microscope (Zeiss, catalog number: 435063-9010-000)
Incubators for C. elegans (15–25°C)
Ethanol candle (DWK Life Sciences, catalog number: 04-245-1)
Autoclave
pH meter (Thermo Electric Corporation)
Fisher Scientific Isotemp Stirrer (Fisher Scientific, catalog number: 11-100-49S)
Pasteur pipette (Fisher Scientific, catalog numer: 22-378893)
Soft and thin eyebrow hair
Tape
Second counter (such as a YouTube video or app that beeps every second)
Platinum wire worm pick (made in-house as described in Wollenberg et al., 2013)
Software
GraphPad Prism v8.4.3. (GraphPad Software, Inc.)
BioRender (www.biorender.com)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Walker, A. C., Bhargava, R., Brust, A. S., Owji, A. A. and Czyz, D. M. (2022). Time-off-pick Assay to Measure Caenorhabditis elegans Motility. Bio-protocol 12(12): e4436. DOI: 10.21769/BioProtoc.4436.
分类
神经科学 > 行为神经科学 > 趋化性
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link