- EN - English
- CN - 中文
Immunomagnetic Isolation and Enrichment of Microvascular Endothelial Cells from Human Adipose Tissue
人体脂肪组织微血管内皮细胞的免疫磁分离和富集
发布: 2022年05月20日第12卷第10期 DOI: 10.21769/BioProtoc.4422 浏览次数: 3445
评审: Giusy TornilloZheng Zachory WeiAnonymous reviewer(s)
Abstract
Human adipose tissue-resident microvascular endothelial cells are not only garnering attention for their emergent role in the pathogenesis of obesity-related metabolic disorders, but are also of considerable interest for vascular tissue engineering due, in part, to the abundant, accessible, and uniquely dispensable nature of the tissue. Here, we delineate a protocol for the acquisition of microvascular endothelial cells from human fat. A cheaper, smaller, and simpler alternative to fluorescence-assisted cell sorting for the immunoselection of cells, our protocol adapts magnet-assisted cell sorting for the isolation of endothelial cells from enzymatically digested adipose tissue and the subsequent enrichment of their primary cultures. Strategies are employed to mitigate the non-specific uptake of immunomagnetic microparticles, enabling the reproducible acquisition of human adipose tissue-resident microvascular endothelial cells with purities ≥98%. They exhibit morphological, molecular, and functional hallmarks of endothelium, yet retain a unique proteomic signature when compared with endothelial cells derived from different vascular beds. Their cultures can be expanded for >10 population doublings and can be maintained at confluence for at least 28 days without being overgrown by residual stromal cells from the cell sorting procedure. The isolation of human adipose tissue-resident microvascular endothelial cells can be completed within 6 hours and their enrichment within 2 hours, following approximately 7 days in culture.
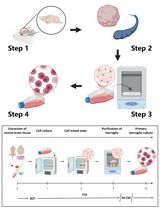
Graphical abstract:
Background
White adipose tissue is not only the predominant store of energy in the body, but it is also an essential endocrine organ that helps regulate systemic metabolic homeostasis (Stern et al., 2016). While the importance of its resident microvascular endothelial cells for the growth and remodeling of the tissue is well-established, their role in the pathogenesis of obesity-related metabolic disorders is only beginning to be appreciated (Graupera et al., 2018). Our robust protocol for the acquisition of human adipose tissue-derived microvascular endothelial cells (HAMVECs) may facilitate these important investigations at the interface of vascular biology and systemic metabolic homeostasis (Antonyshyn et al., 2021). Moreover, it provides the field of vascular tissue engineering with an abundant, accessible, and uniquely dispensable source of endothelial cells, which may ultimately be used for the vascularization of engineered tissues (Rouwkema et al., 2016) and the endothelialization of small diameter vascular prostheses in an autologous, patient-specific manner (Antonyshyn et al., 2020).
Our protocol employs magnet-assisted cell sorting for the immunoselection of HAMVECs (Antonyshyn et al., 2021). Its low cost, small physical footprint, and ease-of-use make it more amenable for a number of stakeholders when compared with alternatives such as fluorescence-assisted cell sorting. Previous procedures for the acquisition of endothelial cells by magnet-assisted cell sorting have relied on differential adhesion (Hutley et al., 2001), clonal selection (Alphonse et al., 2015), and manual weeding (McGinn et al., 2004) to prevent the overgrowth of their primary cultures by residual stromal cells from the cell sorting procedure. These techniques are labour-intensive, time-consuming, and of uncertain reproducibility. We developed a procedure that does not necessitate these additional techniques to establish temporally stable cultures of HAMVECs (Antonyshyn et al., 2021).
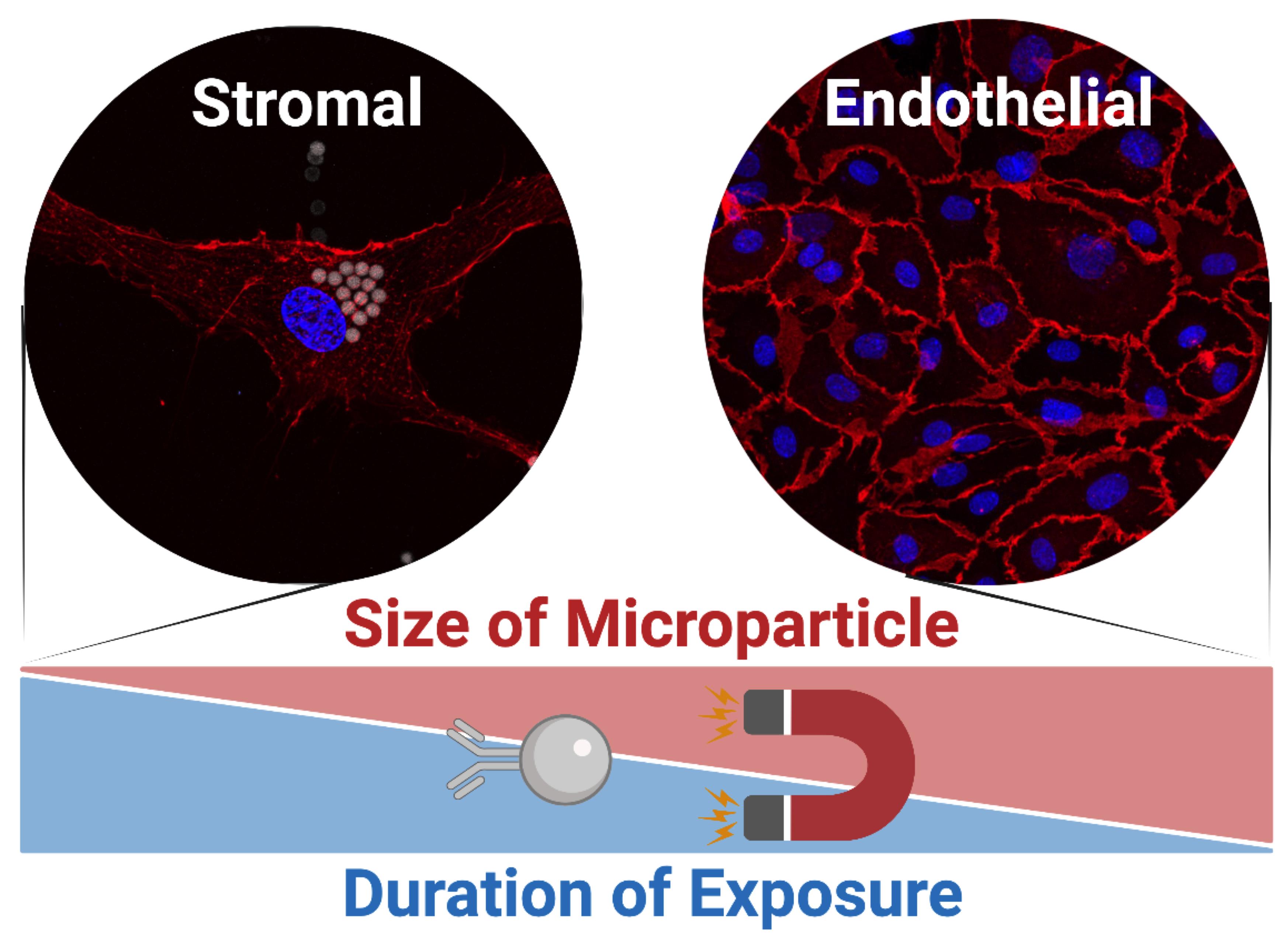
Here, we delineate our straightforward two-staged procedure for the immunomagnetic acquisition of microvascular endothelial cells from human adipose tissue (Antonyshyn et al., 2021). The first step involves the isolation of the endothelial cells from enzymatically digested fat, and the second step involves the enrichment of their primary cultures to eliminate residual adipose tissue-derived stromal/stem cells (ASCs) from the cell sorting procedure. The stromal vascular fraction of enzymatically digested fat is depleted of CD45+ leukocytes prior to positively selecting for CD45–CD31+ HAMVECs. This sequential immunomagnetic selection is used to address challenges arising from the high prevalence of leukocytes in adipose tissue and their capacity to express characteristic endothelial markers, including CD31. The magnet-assisted cell sorting procedure employs immunomagnetic microparticles of a specific size and binding moiety, specifically comprising monodispersed superparamagnetic spheres of a 4.8 µm modal diameter conjugated with antibodies via cleavable deoxyribonucleic acid (DNA) linkers. This defined size distribution of immunomagnetic microparticles prevents their non-specific uptake by ASCs during the labeling portion of the cell sorting procedure, and the cleavable DNA linkers enable the exclusion of the superparamagnetic spheres from primary cultures where they can be more readily internalized by any residual ASCs. These are strategies that mitigate the non-specific uptake of immunomagnetic microparticles by ASCs—which is imperative for the effective isolation and enrichment of endothelial cells by magnet-assisted cell sorting. This facile two-staged procedure enables the reproducible acquisition of HAMVECs with purities ≥98%, which is sufficient to prevent their overgrowth by ASCs and facilitate their use for downstream investigations and regenerative medicine applications. Moreover, the CD45–CD31– ASCs may also be retained for downstream investigations (Antonyshyn et al., 2019), having been previously validated to meet the phenotypic criteria delineated by the International Federation for Adipose Therapeutics and Science and the International Society for Cellular Therapy (Bourin et al., 2013).
Materials and Reagents
Materials
Pipette tips (Corning, Axygen, catalogue numbers: T-300, T-200-Y, and T-1000-B)
Serological pipettes (Corning, Falcon, catalogue numbers: 357543, 357551, and 357525)
Petri dishes, 100 × 15 mm (Fisherbrand, catalogue number: FB0875712)
Tissue culture-treated polystyrene (Corning, Falcon, catalogue numbers: 353043 [12-well plate], 353046 [6-well plate], 353108 [T-25 flask], 353136 [T-75 flask], and 353112 [T-175 flask])
Conical tubes, 15 mL and 50 mL (Corning, Falcon, catalogue numbers: 352096 and 352070)
Round-bottom test tubes, 5 mL (Corning, Falcon, catalogue numbers: 352058)
Steritop Quick Release (0.22 µm vacuum filter; MilliporeSigma, catalogue number: S2GPT05RE)
Millex-GP Syringe Filter Unit (0.22 µm syringe filter; MilliporeSigma, catalogue number: SLGP033RS)
Syringes, 30 mL, BD Luer-Lok tip (Becton, Dickinson and Company, catalogue number: 309650)
Cell Strainers, 40 µm and 100 µm (Fisherbrand, catalogue number: 22-363-547 and 22-363-549)
Reagents
Milli-Q water (MQH2O; from Milli-Q Direct Water Purification System, see Equipment)
Dulbecco’s phosphate-buffered saline, without calcium chloride and magnesium chloride (PBS–/–; MilliporeSigma, catalogue number: D8537)
Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA; MilliporeSigma, catalogue number: E513)
Sodium hydroxide (NaOH; MilliporeSigma, catalogue number: S5881)
Bovine serum albumin solution, 35% (35% BSA stock; MilliporeSigma, catalogue number: A7979)
Ammonium chloride (NH4Cl; MilliporeSigma, catalogue number: A9434)
Potassium bicarbonate (KHCO3; MilliporeSigma, catalogue number: 60339)
Sodium bicarbonate (NaHCO3; MilliporeSigma, catalogue number: S5761)
Krebs-Ringer bicarbonate buffer (KRB powder; MilliporeSigma, catalogue number: K4002)
D-(+)-glucose (MilliporeSigma, catalogue number: G7021)
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; MilliporeSigma, catalogue number: H4034)
Collagenase from Clostridium histolyticum, type II (collagenase, type II; MilliporeSigma, catalogue number: C6885)
Trypsin-EDTA solution (MilliporeSigma, catalogue no. number: T4049)
TrypLE Express (Gibco, catalogue number: 12604013)
Endothelial Cell Growth Medium-2 BulletKit (Lonza, catalogue number: CC-3162)
Biotinylated anti-CD31 antibodies (Miltenyi Biotec, catalogue number: 130-119-893)
CELLection Biotin Binder Kit (anti-biotin Dynabeads; Invitrogen, catalogue number: 11533D)
Dynabeads CD45 (anti-CD45 Dynabeads; Invitrogen, catalogue number: 11153D)
0.5 M EDTA Stock (see Recipes)
Magnet-assisted cell sorting (MACS) Buffer (see Recipes)
Erythrocyte Lysis Buffer (see Recipes)
KRB Stock (see Recipes)
Collagenase Solution (see Recipes)
Equipment
Pipettes (e.g., Eppendorf, catalogue number: 3123000900)
Pipette gun (e.g., Hirschmann Laborgeräte, catalogue number: 9907200)
Scoopula (e.g., Fisherbrand, catalogue number: 14-357Q)
Tissue Forceps, 1 × 2 teeth, 5.75” (e.g., Integra LifeSciences, catalogue number: ST6-44)
Operating Scissors, straight, sharp-blunt, 5.75” (e.g., Integra LifeSciences, catalogue number: 5-SC-16)
Analytical balance (e.g., Mettler Toledo, catalogue number: 1115010657)
pH meter (e.g., VWR, SB20 sympHony; discontinued)
Heated magnetic stir plate (e.g., IKA Works, catalogue number: 0005020001)
Analog vortex mixer (e.g., VWR, catalogue number: 10153-808)
Milli-Q Direct Water Purification System (e.g., MilliporeSigma, catalogue number: ZR0Q008WW)
Biosafety cabinet (e.g., Thermo Fisher Scientific, catalogue number: 1375)
Vacuum pump (e.g., MilliporeSigma, catalogue number: WP6111560)
CO2 incubator (e.g., Thermo Fisher Scientific, catalogue number: 3310)
Centrifuge (e.g., Eppendorf, catalogue number: 022628113)
Transmission light microscope (e.g., Leica Microsystems, catalogue number: 090-135.001)
Water bath (e.g., VWR, catalogue number: 10128-128)
Incubated shaker plate (e.g., VWR, catalogue number: 97009-894)
Magnetic stir bar (e.g., Fisherbrand, catalogue number: 14-512-127)
Beaker, 1 L (e.g., Fisherbrand, catalogue number: FB1001000)
DynaMag-5 Magnet (Invitrogen, catalogue number: 12303D)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Antonyshyn, J. A., Mazzoli, V., McFadden, M. J., Gramolini, A. O., Hofer, S. O. P., Simmons, C. A. and Santerre, J. P. (2022). Immunomagnetic Isolation and Enrichment of Microvascular Endothelial Cells from Human Adipose Tissue. Bio-protocol 12(10): e4422. DOI: 10.21769/BioProtoc.4422.
分类
生物工程 > 生物医学工程
医学 > 心血管疾病
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link