- EN - English
- CN - 中文
Flow Cytometry-based Measurement of Reactive Oxygen Species in Cyanobacteria
基于流式细胞仪的蓝藻活性氧测量
发布: 2022年05月20日第12卷第10期 DOI: 10.21769/BioProtoc.4417 浏览次数: 3829
评审: Dennis J NürnbergAnonymous reviewer(s)

相关实验方案
结核分枝杆菌感染小鼠肺和支气管肺泡灌洗样品中髓样细胞的流式细胞仪分析和荧光激活细胞分选
Alissa C. Rothchild [...] Alan H. Diercks
2020年05月20日 7203 阅读
Abstract
Cyanobacteria are Gram-negative oxygen-producing photosynthetic bacteria that are useful in the pharmaceutical and biofuel industries. Monitoring of oxidative stress under fluctuating environmental conditions is important for determining the fitness, survival, and growth of cyanobacteria in the laboratory as well as in large scale cultivation systems. Here, we provide a protocol developed using unicellular Synechococcus elongatus PCC 7942 and filamentous Fremyella diplosiphon BK14 cyanobacteria for high-throughput oxidative stress measurement by 2′,7′-dichlorodihydrofluorescein-diacetate (DCFH-DA) and flow cytometry (FCM). We also provide details for the optimization of cell number, dye concentration, and FCM parameters for each organism before it can be utilized to quantify reactive oxygen species (ROS). FCM-based method can be used to measure ROS in a large population of cyanobacterial cells in a high-throughput manner.
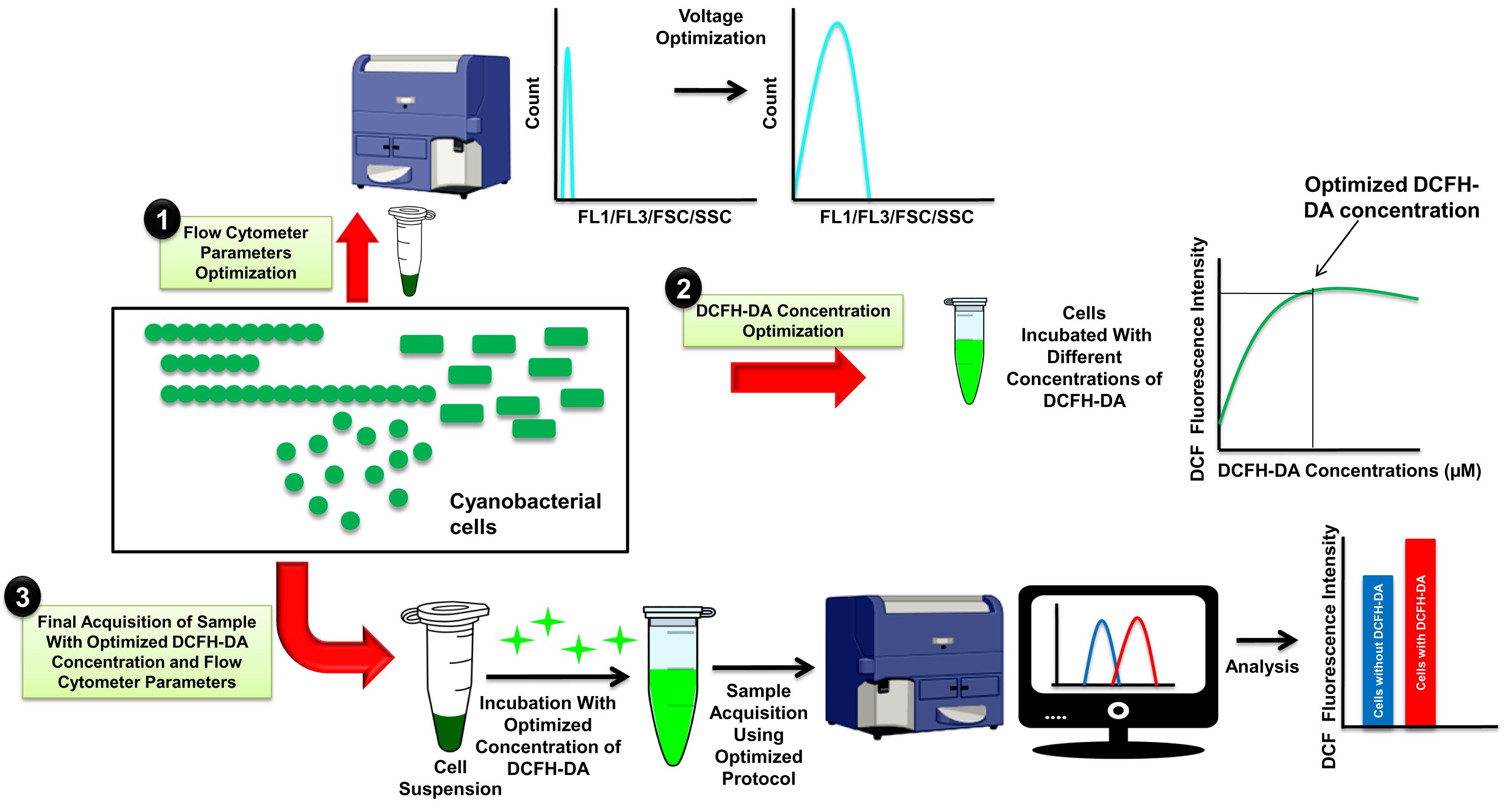
Graphical abstract:
Background
Cyanobacteria are a monophyletic group of Gram-negative bacteria that are found in a variety of habitats and produce oxygen similar to higher plants during photosynthesis (Dvořák et al., 2017). Cyanobacteria have evolved different mechanisms to adapt to a wide range of environmental conditions. These ecologically important organisms are well-known for their significant contribution to global carbon dioxide and nitrogen fixation, and as a result, they contribute significantly to the productivity of ecosystems (Kanno et al., 2017). Cyanobacteria have shown their potential in bioenergy and valuable chemical production due to their minimal growth requirements, high photosynthetic efficiency, amenability to genetic modification, and installation of novel metabolic pathways in their primary metabolic chassis (Rajneesh et al., 2017a). However, oxidation and reduction processes related to photosynthesis and respiration are affected by changing environmental conditions such as light quality and quantity, pH, salinity, temperature, and nutrient limitation. Altered oxidation and reduction processes in cyanobacterial thylakoid membranes result in generation of reactive oxygen species (ROS), which consequently cause oxidative stress (Niyogi, 1999). Increased levels of ROS in cyanobacteria are known to damage lipids, DNA, RNA, pigments, and proteins. ROS also results in photoinhibition due to damage to the oxygen-evolving complex and the D2 protein of photosystem II (Niyogi, 1999; Latifi et al., 2009). As a result, the development, growth, and survival of cyanobacterial cells are impaired by high levels of ROS that are generated under fluctuating environmental conditions (Niyogi, 1999; Latifi et al., 2009). Therefore, rapid and accurate methods to measure ROS are required for monitoring the health of cyanobacterial cells in cultivation systems that are used in commercial setups as well as in basic biological studies.
Earlier, we developed a method to measure ROS levels in cyanobacteria using 2′,7′-dichlorodihydrofluorescein-diacetate (DCFH-DA), a live cell-permeable fluorescent probe that can be visualized by fluorescence microscopy (Rastogi et al., 2010; Rajneesh et al., 2017b). DCFH-DA is a well-known fluorescent probe for detecting ROS in live cells. It hydrolyzes inside the cell to DCFH by the activity of esterases. DCFH cannot cross the cell membrane, and it is mostly non-fluorescent, but it emits green fluorescence when oxidized to dichlorofluorescein (DCF) by intracellular ROS (Kalyanaraman et al., 2012). The intensity of green fluorescence can be measured at 530 nm after excitation at 485 nm. Previous methods have been widely used to measure ROS levels in various organisms in addition to cyanobacteria (Rastogi et al., 2010; Rajneesh et al., 2017b; Li et al., 2017; Basso et al., 2018). However, despite its wide application, fluorescence microscopy-based methods are limited to small sample sizes (the number of cells analyzed is an individual choice, but analysis of at least 50 cells per replicate is recommended), and therefore, the entire population is not well represented (Mondal et al., 2020). Also, imaging a large number of cells in the darkroom is not easy as it is a time-consuming process, and longer exposure of samples to excitation light could result in a high background of green fluorescence due to photooxidation of the dye. Regular imaging for longer periods can also cause dry, itchy, and weary eyes, as well as headaches. Cell morphology is another disadvantage of fluorescence microscopy-based ROS monitoring, as visualizing and estimating ROS in small spherical shape cells is not possible (Mondal et al., 2020). However, FCM-based ROS-monitoring methods overcome the abovementioned limitations of fluorescence microscopy-based methods and provide a high-throughput platform to monitor the ROS in a large number of cells of various morphology. Furthermore, it allows simultaneously recording data on cell size and shape, granularity, chlorophyll, and phycobiliproteins while monitoring the ROS levels. The forward scatter (FSC) parameter of FCM provides information about cell size and shape, while the internal complexity, i.e., granularity, of a cell can be determined by measuring side scatter. Granules and the nucleus are two cellular components that influence side scatter; however, the nucleus is absent in cyanobacteria. Similar to fluorescence microscopy-based ROS-detection methods described earlier (Rastogi et al., 2010; Rajneesh et al., 2017b), this protocol can be optimized for different organisms for successful monitoring of ROS.
Materials and Reagents
1.5 mL amber microcentrifuge tubes (Abdos, catalog number: P10204A)
3 mL round bottom polystyrene tubes (Becton Dickinson, catalog number: 156758)
20 mL 1 M HEPES pH 8.0 (Himedia, catalog number: RM380-500G)
Glass slides (Borosil, catalog number: BT409100P02)
Cover slips (Borosil, catalog number: 9115S01)
1 mL pipette tips (Abdos, catalog number: P10109)
200 µL pipette tips (Abdos, catalog number: P10140)
10 µL pipette tips (Abdos, catalog number: P10116)
1 cm quartz cuvette (Shimadzu, catalog number:226-85010-92)
0.2 µm size Millipore membrane filter (Merck, catalog number: GSWP04700)
BD FACS Clean Solution (0.1% Sodium hypochlorite) (Becton Dickinson, catalog number: 340345)
Hydrogen Peroxide (Merck, catalog number: 1.93408.0521)
Milli-Q water
Exponentially growing cultures of Synechococcus elongatus PCC 7942, Synechocystis sp. PCC 6803 and Fremyella diplosiphon BK14 grown in BG11 medium supplemented with 20 mM HEPES (Allen, 1968).
Note: Synechococcus elongatus PCC 7942, Synechocystis sp. PCC 6803, and Fremyella diplosiphon BK14 (Kehoe and Grossman, 1996) were grown in BG11 liquid medium supplemented with 20 mM HEPES. Cells were inoculated from a solid BG11+20 mM HEPES agar plate. Synechococcus elongatus PCC 7942 and Synechocystis sp. PCC 6803 were grown under PAR (photosynthetically active radiation) (~80 µmol m-2 s-1), while Fremyella diplosiphon BK14 was grown in red light (Red LED Fluorescent light, Havells, India) (~20 µmol m-2 s-1) with ~150 rpm shaking at 25°C. The growth curves of all organisms were monitored, and cultures were maintained in the exponential phase by regular sub-culturing.
DCFH-DA (Sigma-Aldrich, catalog number: D6883)
Ethanol (Merck, catalog number: 1.00983.0511)
Disodium hydrogen phosphate (Na2HPO4) (Merck, catalog number: 1.93622.0521)
Sodium dihydrogen phosphate (NaH2PO4) (Merck, catalog number: 1.93624.0521)
Sodium chloride (NaCl) (SRL, catalog number: 41721)
2 mM (w/v) DCFH-DA Stock solution (1 mL) (see Recipes)
0.1 M PBS pH 7.4 (100 mL) (see Recipes)
Equipment
BD FACSCalibur flow cytometer (Becton Dickinson, catalog number: 342975)
UV-VIS Spectrophotometer 1800 (Shimazdu, catalog number: 80626)
Nikon 90i eclipse fluorescence microscope (Nikon, Japan)
Cooling Centrifuge (Remi, India/NEYA16R)
Software
BD CellQuest Pro (Becton Dickinson, USA)
NIS elements AR software 4.0 (Nikon, Japan)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Mondal, S. and Singh, S. P. (2022). Flow Cytometry-based Measurement of Reactive Oxygen Species in Cyanobacteria. Bio-protocol 12(10): e4417. DOI: 10.21769/BioProtoc.4417.
分类
微生物学 > 微生物生理学 > 胁迫反应
植物科学 > 藻类学 > 生理学
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link