- EN - English
- CN - 中文
Low-viscosity Matrix Suspension Culture for Human Colorectal Epithelial Organoids and Tumoroids
用于人结直肠上皮类器官和类肿瘤的低粘度基质悬浮培养
发布: 2022年04月20日第12卷第8期 DOI: 10.21769/BioProtoc.4394 浏览次数: 4044
评审: Tomohiro MizutaniJatin RoperAnonymous reviewer(s)

相关实验方案
来自骨髓增生性肿瘤患者的造血祖细胞的血小板生成素不依赖性巨核细胞分化
Chloe A. L. Thompson-Peach [...] Daniel Thomas
2023年01月20日 2360 阅读
Abstract
Three-dimensional culture of human normal colorectal epithelium and cancer tissue as organoids and tumoroids has transformed the study of diseases of the large intestine. A widely used strategy for generating patient-derived colorectal organoids and tumoroids involves embedding cells in domes of extracellular matrix (ECM). Despite its success, dome culture is not ideal for scalable expansion, experimentation, and high-throughput screening applications. Our group has developed a protocol for growing patient-derived colorectal organoids and tumoroids in low-viscosity matrix (LVM) suspension culture. Instead of embedding colonic crypts or tumor fragments in solid ECM, these are grown suspended in medium containing only a low percentage of ECM. Compared with dome cultures, LVM suspension culture reduces the labor and cost of establishing and passaging organoids and tumoroids, enables rapid expansion, and is readily adaptable for high-throughput screening.
Graphical abstract:
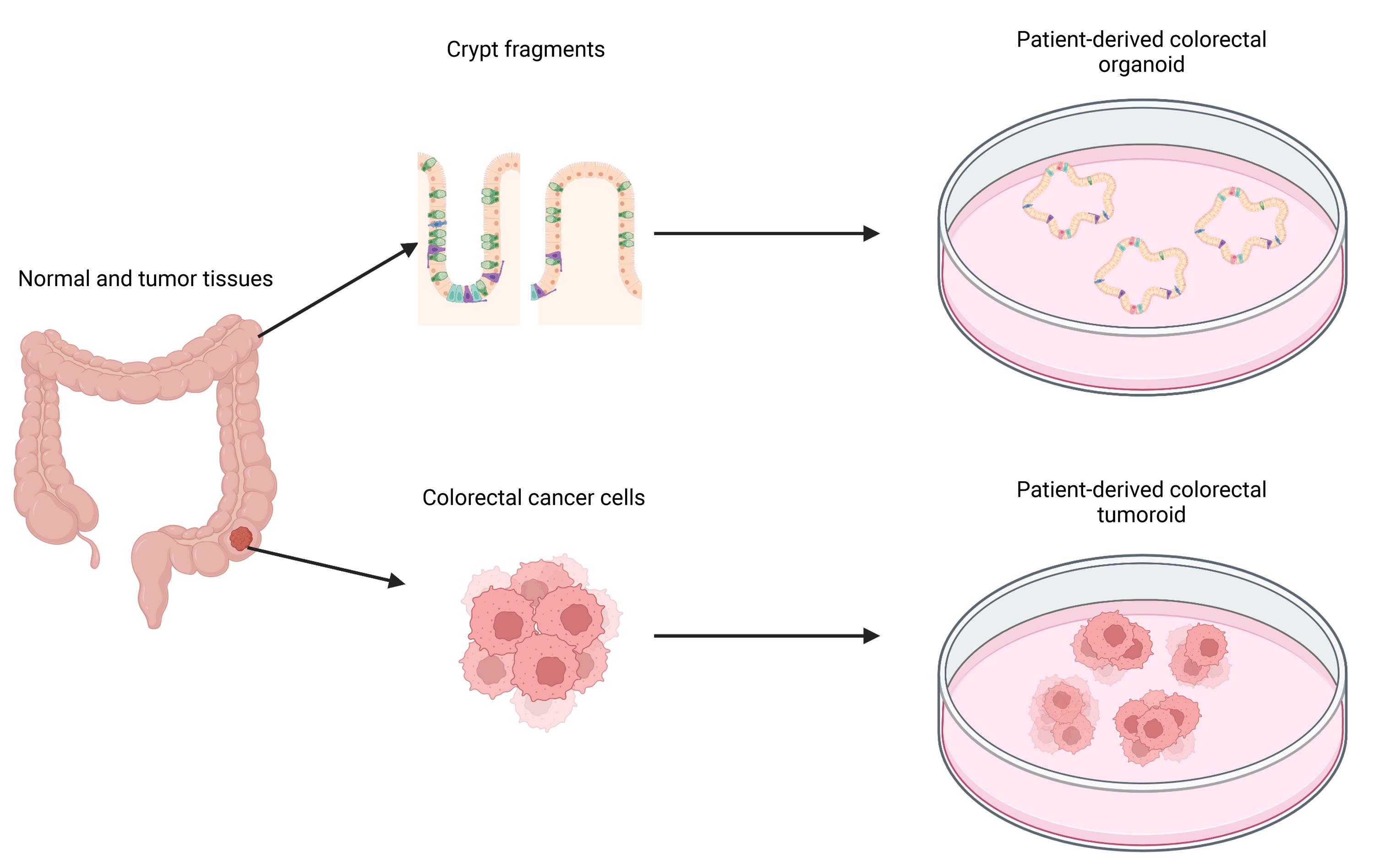
Generation of organoids and tumoroids from human large intestine using LVM suspension culture (Created with BioRender.com).
Background
Patient-derived three-dimensional in vitro tissue models of normal and cancer tissues, referred to as organoids and tumoroids, have become an invaluable tool for the study of disease biology, and the development of precision medicine approaches. Organoids and tumoroids can be established from colorectal crypts and tumor fragments embedded in extracellular matrix (ECM), with medium containing growth factors and inhibitors supporting the growth of intestinal stem cells (Pinto et al., 2003; Kuhnert et al., 2004; Jung et al., 2011; Sato et al., 2011; Weeber et al., 2015). While embedment into solid ECM is considered essential for intestinal epithelial organoid and tumoroid culture (Sato et al., 2011), this is associated with technical challenges that hamper culture expansion and experimentation. Organoid and tumoroid growth in solid matrices is limited by the accumulation of solid stress, as well as the delivery of oxygen and nutrients (Roose et al., 2003), requiring frequent passaging to enable culture maintenance and expansion. In addition, solid matrices must be removed mechanically or using enzymes to isolate organoids and tumoroids for propagation or downstream experimental applications (Sato et al., 2011).
To address the limitations of organoid and tumoroid culture in solid ECM, we have developed a low-viscosity matrix (LVM) suspension culture method. Instead of ECM embedment, organoids and tumoroids are grown suspended in culture medium containing only a low percentage of ECM, reducing labor and cost. We have demonstrated the utility of our LVM suspension culture approach for organoid and tumoroid establishment, propagation, scalable expansion, and biobanking, as well as tumoroid high-throughput drug testing, as detailed in our related research article (Hirokawa et al., 2021).
Materials and Reagents
Corning Tissue-culture treated culture Petri dishes 100 × 20 mm (Merck, catalog number: CLS430167)
Pipettes
Axygen filter tips 10 μL, 20 μL, 200 μL, 1,000 μL (Corning, catalog numbers: TF-1000-L-R-S, TF-200-L-R-S, TF-20-L-R-S, TF-10-L-R-S)
Greiner Bio-One 6 Well Suspension Culture Plates (Interpath, catalog number: 657185)
CorningTM Mini Bioreactor Centrifuge Tubes (Corning, catalog number: 431720)
Nunc 50 mL Conical Sterile Centrifuge Tubes (Thermo Fisher Scientific, catalog number: 339652)
Centrifuge tube 10 mL 100 × 16 mm (Sarstedt, catalog number: 62.9924.284)
Tube Micro 1.5 mL Centrifuge Polypropylene Clear Autoclaved (Eppendorf, catalog number: 0030121872)
NalgeneTM Rapid-FlowTM Sterile Single Use Vacuum Filter Units (Thermo Fisher Scientific, catalog number: 564-0020)
Human IntestiCult Organoids Growth Medium (Stemcell Technologies, catalog number: 6010)
Primocin (Invivogen, catalog number: ant-pm-1)
Penicillin-Streptomycin (10,000 U/mL) (Life Technologies, catalog number: 15140122)
GibcoTM Gentamicin (50 mg/mL) (Thermo Fisher Scientific, catalog number: 15750078)
Gibco Advanced DMEM/F-12 (Thermo Fisher Scientific, catalog number: 12634028)
Gibco GlutaMAXTM Supplement (Thermo Fisher Scientific, catalog number: 35050061)
HEPES (Sigma-Aldrich, catalog number: H3375-1KG)
Nicotinamide (Sigma-Aldrich, catalog number: 72340-1KG)
N-Acetyl-l-Cysteine (Sigma-Aldrich, catalog number: A7250-1KG)
B-27 Supplement (50×) (Life Technologies, catalog number: 17504001)
N-2 Supplement (100×) (Life Technologies, catalog number: 17502001)
Normocin (Invivogen, catalog number: ant-nr-2)
Human recombinant basic FGF (FGF2) (Life Technologies, catalog number: PHG0263)
Human recombinant EGF (Life Technologies, catalog number: PHG0313)
Y-27632 (Stemcell Technologies, catalog number: 72308)
Corning® Matrigel® Basement Membrane Matrix, *LDEV-Free, 10 mL (Corning, catalog number: 356234)
Dispase II (protease) (Merck, catalog number: D4693-1G)
Gibco Collagenase IV (Life Technologies, catalog number: 17104019)
TrypLE Express Enzyme (1×) (Thermo Fisher Scientific, catalog number: 12604021)
CryoStor® CS10 (Stemcell Technologies, catalog number: 07930)
Dulbecco's Phosphate Buffered Saline (Life Technologies, catalog number: 14040182)
Gibco DMEM/F12 (Life Technologies, catalog number: 11320082)
Gibco DMEM/F12 + GLUTAMAX (Life Technologies, catalog number: 10565042)
Ethylenediaminetetraacetic acid (EDTA) (Merck, catalog number: E9884)
Dithiothreitol (DTT) (Merck, catalog number: 11583786001)
A83-01 (Tocris Bioscience, catalog number: 2939)
SB202190 (Sigma-Aldrich, catalog number: S7067)
Bovine serum albumin (BSA) (Merck, catalog number: A9418)
Anti-Adherence Rinsing Solution (Stemcell Technologies, catalog number: 07010)
Tissue medium (see Recipes)
EDTA chelation buffer (see Recipes)
Colorectal tumoroid primary culture medium (see Recipes)
Colorectal tumoroid maintenance medium (see Recipes)
Equipment
Biosafety cabinet (Tissue culture hood)
Dissection Forceps and Scissors
Corning CoolCell LX Cell Freezing Container (Biocision, model: BCS-405)
Benchtop Centrifuge 5424 (Eppendorf, model: 5424)
Tabletop Centrifuge 5810R (Eppendorf, model: 5810R)
Bright-field inverted Tissue Culture Microscope
HERAcell VIOS 160i CO2 incubator (Thermo Fisher Scientific, catalog number: HEA51030287)
Ultra low freezer (-80°C)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Tan, T., Hirokawa, Y., Clarke, J., Sakthianandeswaren, A. and Sieber, O. M. (2022). Low-viscosity Matrix Suspension Culture for Human Colorectal Epithelial Organoids and Tumoroids. Bio-protocol 12(8): e4394. DOI: 10.21769/BioProtoc.4394.
分类
癌症生物学 > 癌症干细胞 > 细胞生物学试验 > 细胞分离和培养
细胞生物学 > 细胞分离和培养 > 3D细胞培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link