- EN - English
- CN - 中文
Apoplastic Expression of CARD1-ecto Domain in Nicotiana benthamiana and Purification from the Apoplastic Fluids
本氏烟草中 CARD1-ecto 结构域的质外体表达及质外体液的纯化
发布: 2022年04月20日第12卷第8期 DOI: 10.21769/BioProtoc.4387 浏览次数: 3554
评审: Ashish RanjanMalgorzata LichockaDemosthenis ChronisShweta Panchal

相关实验方案
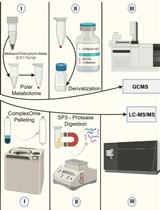
利用SP3珠和稳定同位素质谱技术优化蛋白质合成速率:植物核糖体的案例研究
Dione Gentry-Torfer [...] Federico Martinez-Seidel
2024年05月05日 2852 阅读
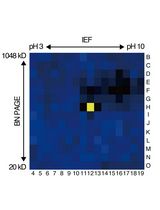
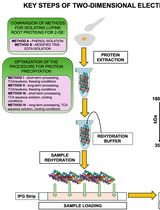
基于活性蛋白质组学和二维聚丙烯酰胺凝胶电泳(2D-PAGE)鉴定拟南芥细胞间隙液中的靶蛋白酶
Sayaka Matsui and Yoshikatsu Matsubayashi
2025年03月05日 1966 阅读
Abstract
The protein expression and purification process is an essential initial step for biochemical analysis of a protein of interest. Traditionally, heterologous protein expression systems (such as E. coli, yeast, insect cells, and cell-free) are employed for plant protein expression, although a plant expression system is often desirable for plant proteins, to ensure proper post-translational modifications. Here, we describe a method to express and purify the ectodomain of one of the leucine-rich repeat receptor-like kinase called CARD1/HPCA1, from Nicotiana benthamiana apoplastic fluid. First, we express His-tagged CARD1 ectodomain in the apoplastic space of N. benthamiana by the Agroinfiltration method. Then, we collect apoplastic fluids from the leaves and purify the His-tagged protein by Ni2+-affinity chromatography. In addition to plant-specific post-translational modifications, protein accumulated in the plant apoplastic space, rather than in the cytosolic space, should be kept under an oxidizing environment. Such an environment will help to maintain the property of intrinsic disulfide bonds in the protein of interest. Further, purification from the apoplastic fluids, rather than the total protein extract, will significantly reduce contaminants (for instance RuBisCO) during protein extraction, and simplify downstream processes. We envisage that our system will be useful for expressing various plant proteins, particularly the apoplastic or extracellular regions of membrane proteins.
Keywords: Protein expression and purification (蛋白质表达和纯化)Background
The use of Nicotiana benthamiana as a host organism for protein expression has increasingly become an attractive system, in addition to well-established expression systems like E. coli, yeast, insect cells, and cell-free. However, it is challenging to obtain near homogeneity protein by one-step affinity purification in the N. benthamiana system, possibly due to the complexity of the total protein extract (Souza, 2015). An alternative option is to ensure that the proteins of interest are expressed and accumulated in specialized compartments, which are spatially separated from contaminants derived from other compartments. Thus, by collecting proteins of interest accumulated in a specific compartment, we can simplify the heterogeneity of the starting material for column chromatography.
Many plant proteins passing through the secretory pathway need to get oxidized and form disulfide bonds, to mature into a stable form (Meyer et al., 2019). The appropriate disulfide bonding is required in many apoplastic proteins or extracellular regions of membrane proteins, including membrane-bound leucine-rich repeat (LRR) receptor-like kinase, and secreted peptide hormones (Meyer et al., 2019). However, the cytoplasm is normally maintained in a reducing environment. It is desirable to accumulate the expressed proteins in the apoplast, and retrieve them with appropriate disulfide bond modifications.
In this protocol, we describe a method to express and purify the ectodomain of an LRR receptor-like kinase CARD1/HPCA1 (CARD1ecto) from N. benthamiana apoplastic fluid. We will primarily focus on its expression and accumulation in the apoplast (Procedure A), its extraction from the apoplastic space (Procedure B), and its purification by Ni2+-affinity chromatography (Procedure C). This system should prove useful not only for CARD1ecto, but also for other plant proteins of interest, particularly the apoplastic or extracellular regions of membrane proteins, which may require redox-related modifications to function correctly.
Materials and Reagents
1 mL needleless plastic syringe (TERUMO, catalog number: SS-01T)
20 mL needleless plastic syringe (TERUMO, catalog number: SS-20ESz)
50 mL centrifuge tube (FALCON, catalog number: 352070)
GD/X syringe filter (PES 0.45 μm) (GE healthcare, catalog number: 6876-2504)
500 mL Pyrex beaker
300 mL Pyrex beaker
Vacuum desiccator
15 mL centrifuge tube (FALCON, catalog number: 352097)
Soil-grown Nicotiana benthamiana
pEAQ-HT plasmid (Sainsbury et al., 2009)
Agrobacterium tumefaciens C58C1 carrying pCH32 (Hamilton et al., 1996; Hellens et al., 2000)
LB Broth (Lennox) (Sigma-Aldrich, catalog number: L7275-500TAB)
Kanamycin (FUJIFILM Wako Chemicals, catalog number: 113-00343)
Rifampicin (FUJIFILM Wako Chemicals, catalog number: 185-01003)
4'-Hydroxy-3',5'-dimethoxyacetophenone (acetosyringone) (Sigma-Aldrich, catalog number: D134406-25G)
Bis-Tris (Dojindo, catalog number: 6976-37-0)
Tween 20 (polyoxyethylene sorbitan monolaurate) (Nacalai Tesque, catalog number: 35624-15)
cOmpleteTM ULTRA Tablets, EDTA-free, Protease Inhibitor Cocktail (Merck, catalog number: 5892953001)
Sodium Chloride (FUJIFILM Wako Chemicals, catalog number: 195-01663)
Magnesium Chloride (FUJIFILM Wako Chemicals, catalog number: 136-03995)
Immobilized Ni2+-affinity column, HisTrap excel (Cytiva, catalog number: 17371205)
Note: Whilst any standard Ni2+-affinity column should be acceptable, we recommend using the HisTrap excel column by Cytiva. In this method, the His-tagged protein will be purified in buffer at pH 6.0, given that apoplast space is usually weakly acidic. According to the manufacturer’s instructions, HisTrap excel can capture His-tagged proteins even at pH 6.0.
Superloop, 1/16" fittings (ÄKTAdesign), 50 mL (Cytiva, catalog number: 18111382)
Imidazole (FUJIFILM Wako Chemicals, catalog number: 095-00015)
Vivaspin Turbo 15 (10 kDa molecular weight cut off) (Sartorius, catalog number: VST15T01)
Coomassie protein stain, such as InstantBlue (Expedeon, catalog number: ISB01L)
Agroinfiltration buffer (see Recipes)
Vacuum infiltration buffer (see Recipes)
Equilibration buffer (see Recipes)
Wash buffer (see Recipes)
Elution buffer (see Recipes)
Equipment
Vacuum pump (e.g., ULVAC DTC-41, or equivalent model)
Electroporation system (e.g., Bio-Rad Gene Pulser XCellTM, or equivalent)
Centrifuge with a swing rotor for 50 mL centrifuge tubes (e.g., Hitachi CF16RXII with swing rotor T4SS31, or equivalent)
Centrifuge with a fixed angle rotor for 15 mL centrifuge tubes (e.g., Hitachi CR20GIII with fixed angle rotor R15A, or equivalent)
Fast Protein Liquid Chromatography machine (we used the equivalent of GE healthcare AKTA pure 25 M1 (Cytiva, catalog number 29018227), with optional accessories installed).
Note: Any fast protein liquid chromatography machine from companies such as Cytiva (https://www.cytivalifesciences.com/) or from Bio-Rad (https://www.bio-rad.com/) are fine.
Spectrophotometer (e.g.,Thermo Fisher Scientific NanodDrop OneC, or equivalent)
Standard SDS-PAGE equipment (such as Mini-PROTEAN® Tetra Cell from Bio-Rad). For the SDS-PAGE protocol, please refer to Green and Sambrook (2014).
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Ishihama, N., Laohavisit, A., Takizawa, K. and Shirasu, K. (2022). Apoplastic Expression of CARD1-ecto Domain in Nicotiana benthamiana and Purification from the Apoplastic Fluids. Bio-protocol 12(8): e4387. DOI: 10.21769/BioProtoc.4387.
分类
植物科学 > 植物生物化学 > 蛋白质 > 分离和纯化
生物化学 > 蛋白质 > 分离和纯化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link