- EN - English
- CN - 中文
Conditional Human BRD4 Knock-In Transgenic Mouse Genotyping and Protein Isoform Detection
条件性人 BRD4 敲入转基因小鼠基因分型和蛋白质异构体检测
发布: 2022年04月05日第12卷第7期 DOI: 10.21769/BioProtoc.4374 浏览次数: 3617
评审: Gal HaimovichKirsten A. CoprenAnonymous reviewer(s)
Abstract
Bromodomain-containing protein 4 (BRD4) is an acetyl-lysine reader protein and transcriptional regulator implicated in chromatin dynamics and cancer development. Several BRD4 isoforms have been detected in humans with the long isoform (BRD4-L, aa 1-1,362) playing a tumor-suppressive role and a major short isoform (BRD4-S, aa 1-722) having oncogenic activity in breast cancer development. In vivo demonstration of the opposing functions of BRD4 protein isoforms requires development of mouse models, particularly transgenic mice conditionally expressing human BRD4-L or BRD4-S, which can be selectively induced in different mouse tissues in a spatiotemporal-specific manner. Here, we detail the procedures used to genotype transgenic mouse strains developed to define the effects of conditional human BRD4 isoform expression on polyomavirus middle T antigen (PyMT)-induced mouse mammary tumor growth, and the key steps for Western blot detection of BRD4 protein isoforms in those tumors and in cultured cells. With this protocol as a guide, interpretation of BRD4 isoform functions becomes more feasible and expandable to various biological settings. Adequate tracking of BRD4 isoform distributions in vivo and in vitro is key to understanding their biological roles, as well as avoiding misinterpretation of their functions due to improper use of experimental procedures that fail to detect their spatial and temporal distributions.
Graphic abstract:

Background
Bromodomain-containing protein 4 (BRD4) is a member of the bromodomain and extra-terminal (BET) family proteins that also include BRD2, BRD3, and testes/germ cell-specific BRDT (Wu and Chiang, 2007). BET proteins are epigenetic regulators implicated in chromatin dynamics and cancer development (Li et al., 2018; Kim et al., 2019; Wu et al., 2020). BRD4 is typically required for expression of c-Myc (Zuber et al., 2011), FosL1 (Lockwood et al., 2012), and other oncogenes, primarily via enhancer/promoter regulation (Lovén et al., 2013; Wu et al., 2020). With the availability of small-compounds inhibiting BET proteins, particularly BRD4 (Filippakopoulos et al., 2010; Nicodeme et al., 2010; Cai et al., 2011; Chiang, 2014; Tang et al., 2021; Liu et al., 2022), epigenetic therapy targeting BET proteins is closer to reality. Nevertheless, accurate detection of BET proteins in various cell lineages and tissues can be challenging, due to their tight association with chromatin in a confined nuclear compartment (Wu et al., 2006), dependency on BET protein phosphorylation for chromatin association and factor interaction (Chiang, 2016; Wu et al., 2013, 2016), and existence of multiple protein isoforms (Chiang, 2014; Wu et al., 2020). In humans, BRD4 has two universally expressed protein isoforms (Wu and Chiang, 2007) – BRD4-L (long, aa 1-1,362) and BRD4-S (short, aa 1-722) – generated by alternative 3′ splicing. Without proper solubilization and prompt processing of the isolated nuclear samples, BET proteins can be easily degraded or remain bound to chromatin, making it difficult to detect the intact proteins or distinguish the intrinsic smaller protein isoforms from the degradation products of the long isoform.
Using a conditional BRD4 knock-in (KI) transgenic mouse model, we showed that human BRD4-L plays a tumor suppressive role, and BRD4-S has oncogenic activity in breast cancer initiation, progression, and metastasis (Wu et al., 2020). BRD4-L and BRD4-S share common N-terminal amino acid (aa) residues from 1 to 719, with BRD4-S possessing three additional residues (GPA: glycine-proline-alanine) after aa 719, and BRD4-L containing a much longer C-terminal extension (Figure 1). To distinguish BRD4-S from BRD4-L, we have generated isoform-specific (S and L) and common (Pan) BRD4 antibodies. All of these are raised in rabbits, with a 14-aa peptide (S) or purified protein fragments (N and L) as antigens (Figure 1; for detail, see Wu et al., 2006 and 2016).
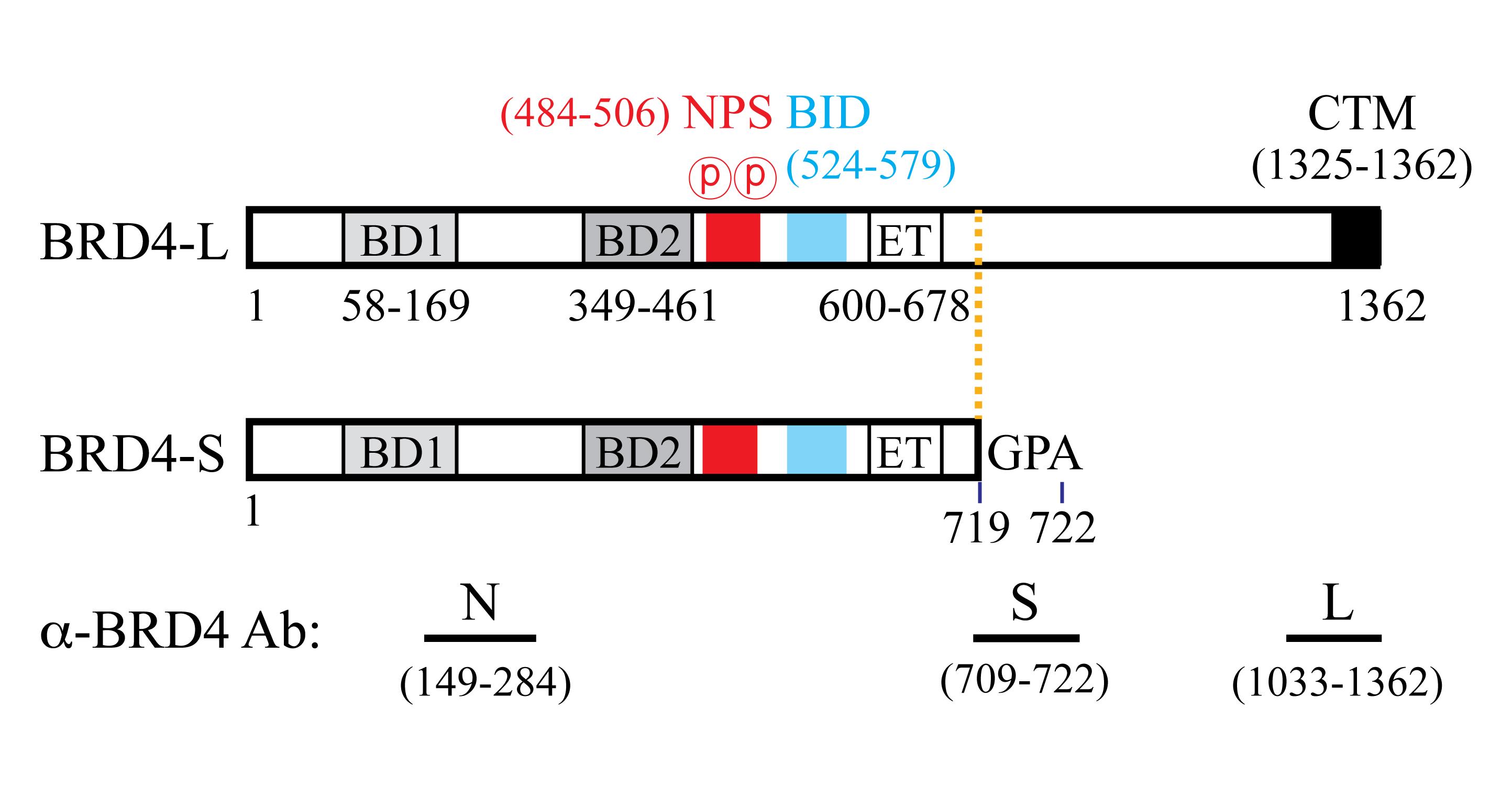
Figure 1. Human BRD4 protein isoform features and antibodies. Abbreviations: BD1, first bromodomain; BD2, second bromodomain; NPS, N-terminal cluster of CK2 phosphorylation sites; BID, basic residue-enriched interaction domain; ET, extra-terminal domain; CTM, C-terminal motif. N is a pan antibody recognizing aa 149–284 commonly found in both BRD4-L and BRD4-S isoforms. Numbers correspond to the positions of the amino acid residues.
The BRD4-KI mice illustrated in this protocol are transgenic rodents, containing either a human BRD4-L or BRD4-S transgene introduced at the Rosa26 (Reverse orientation splice acceptor) locus (Figure 2A; Wu et al., 2020). Expression of the BRD4-L or BRD4-S transgene is controlled by the CAGGS promotor and a loxP-STOP-loxP cassette, where inducible expression of human BRD4 isoforms is triggered by Cre recombinase in a ubiquitous or tissue-specific manner. In the presence of Cre, recombination at the 34-bp loxP elements excises the STOP sequence, and allows the CAGGS promotor to drive BRD4 transgene expression (Figure 2A). Mice harboring human BRD4 transgenes can be crossed with other mouse strains to generate experimental and control groups for specific biological investigation. In this protocol, we utilize a breast cancer model in which BRD4-KI mice are crossed with mice expressing Cre and/or oncogenic PyMT (polyomavirus middle T antigen) in mammary fat pad tissue (see Table 1 for mouse strain information). Genomic DNA from mouse tail is then analyzed to genotype the offspring. The genotyping protocol starts with mouse tail snipping, tail lysis, polymerase chain reaction (PCR), and DNA agarose gel electrophoresis, to ultimately select for experimentation the mice with or without a specific transgene, namely human BRD4, MMTV-Cre, and MMTV-PyMT (Figure 2B). The Western blotting protocol details the important steps used to monitor endogenous mouse (m) and exogenous human (h) BRD4 protein expression, with emphasis on conditions for BRD4 solubilization from chromatin, use of wet protein transfer, and BRD4 isoform-specific (α-BRD4-L and α-BRD4-S) and Pan (α-BRD4-N) antibodies.
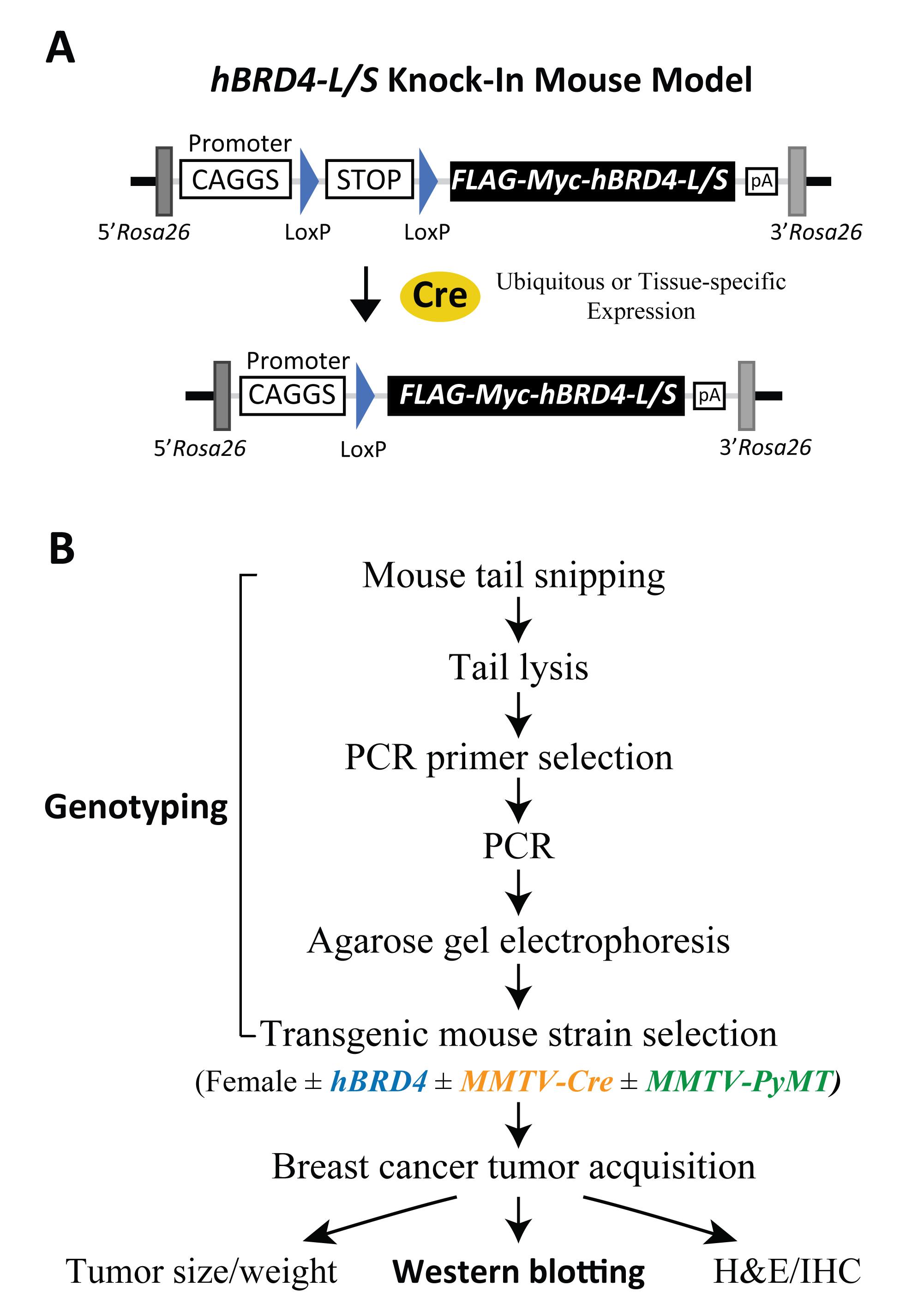
Figure 2. Schematic of human BRD4-L/S knock-in constructs and outline of genotypic and phenotypic analyses. (A) Schematic drawing of hBRD4-L/S knock-in mouse models showing Cre-loxP-dependent recombination that triggers CAGGS promoter-driven expression of the FLAG-Myc-tagged BRD4-L/S transgene located in the Rosa26 gene locus in a ubiquitous or tissue-specific manner. (B) Flowchart detailing the experimental scheme involved in transgenic mouse genotyping from tail snipping and lysis, PCR, agarose gel electrophoresis, and mouse selection for studying PyMT-induced tumor growth with or without Cre-mediated BRD4 transgene expression and downstream phenotypic, protein, and histologic analyses. Abbreviation: MMTV, mouse mammary tumor virus.
The advantage of our genotyping procedure is the unique PCR protocol used to detect the presence of hBRD4-L and hBRD4-S in mice, using human allele-specific primers for amplification of the common or unique coding regions of each BRD4 isoform (Figure 3A for gene maps, and Table 2 for primer information). A PCR primer pair specific to hBRD4-L exons 13 and 15 (see Figure 3A, hBRD4-L, arrows for primer positions) is used to amplify the hBRD4-L transgene, which produces a PCR product of 216 bp (Figure 3B, hBRD4-L gel). To amplify hBRD4-S, we use a forward PCR primer which anneals to common hBRD4 exon 11, and a reverse primer unique to hBRD4-S exon 12 (see Figure 3A, hBRD4-S), yielding a PCR product of 136 bp (Figure 3B, hBRD4-S gel). A quick independent validation of either hBRD4 transgene is performed by using a forward primer that anneals to the FLAG sequence and a reverse primer that recognizes hBRD4 exon 3 (Figure 3A, hBRD4-Pan), producing a PCR product of 520 bp (Figure 3B, hBRD4-Pan gel). The human BRD4 transgene is inserted into the Rosa26 locus located on mouse chromosome 6. To amplify this region, we select a forward and reverse primer pair flanking a provirus integration site of the Rosa26 locus (Figure 3A; Soriano, 1999). Detection of a 600-bp PCR product representing an uninserted locus would indicate no BRD4 transgene, or a heterozygous configuration if concurrent BRD4-L or -S transgene is detected.
To detect the presence of the Cre transgene necessary for hBRD4-L/S expression, a Cre-specific primer pair (He et al., 2003; Wu et al., 2020) is used, generating a PCR product of 374 bp (Figure 3B, MMTV-Cre gel). With this primer pair, the zygosity of Cre cannot be determined; however, for Cre-mediated loxP recombination, a homozygous Cre genotype (Cre/Cre) is not essential (Reference 17). Sometimes, the Cre protein can cause unexpected phenotypes in certain heterozygous MMTV-Cre mouse strains (e.g., line A), such as lactational defects (Yuan et al., 2011), or chromosomal abnormalities in some Cre-overexpressed mice, where DNA damage is induced in postmitotic spermatids possibly due to loxP-like sequence-triggered recombination in the mammalian genome (Schmidt-Supprian and Rajewsky, 2007). Thus, all Cre mouse strains used in this protocol are derived from line D (Wagner et al., 1997), and bred to carry only one copy of the Cre gene for tissue-specific expression, to avoid overexpression-induced defects. Since neither hBRD4-L or hBRD4-S alone is sufficient to induce mouse mammary tumors (our unpublished data), oncogenic PyMT is used to induce tumor formation in the mammary fat pads of female mice (Guy et al., 1992). PyMT exon 2-specific forward and reverse primers (Figure 3A) were used, resulting in a PCR product of 569 bp (Figure 3B, MMTV-PyMT gel). Hemizygous female MMTV-PyMT mice were purchased from the Jackson Laboratory, as they produce sizable mammary fat pad tumors for breast cancer study, so all mice in this protocol are bred to carry only one copy of the MMTV-PyMT gene.
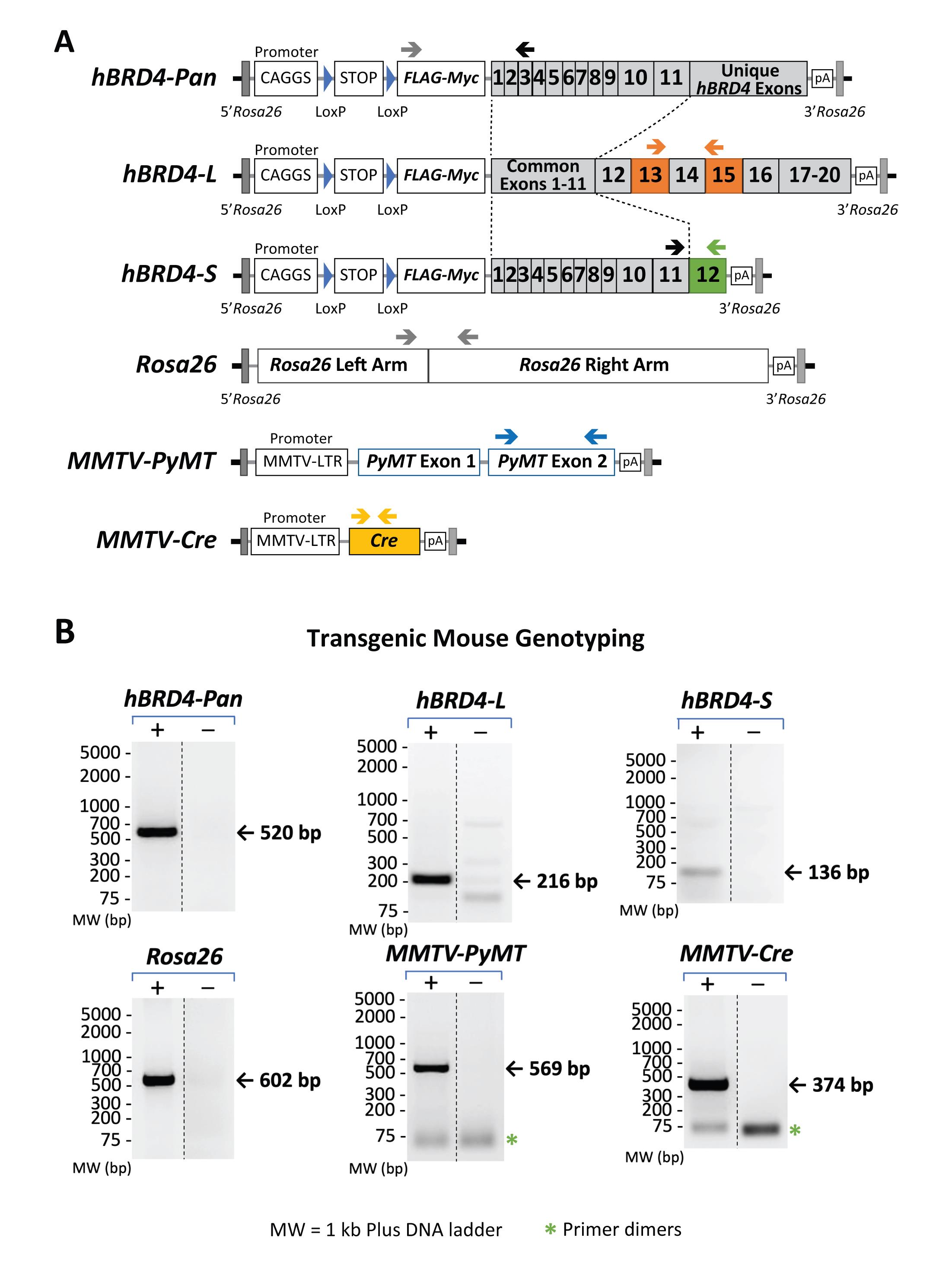
Figure 3. Gene maps showing primer-annealing sites on specific transgenes and the Rosa26 locus, as well as the sizes of expected PCR products. (A) Genomic maps of hBRD4-L, hBRD4-S, MMTV-PyMT, and MMTV-Cre transgenes and the Rosa26 locus. Positions of each PCR primer pair are shown as arrows above each gene map. (B) Agarose gel images showing the specificity of each primer pair and the size of its resulting PCR product. The presence (+) or absence (-) of a transgene, monitored by PCR, is shown in paired gel images (same exposure) from nonadjacent lanes (marked by a vertical dash line) in the same gel. The DNA ladders (in base pairs) are marked on the left. The bands representing primer dimerization (*) are also indicated.
Using the selective PCR strategies outlined above together with proper breeding schemes, hBRD4-KI genotyping can be easily performed to study the effects of conditional hBRD4 isoform expression on cancer initiation and tumor growth. Analysis of various downstream phenotypes, including tumor size/weight measurements, morphological hematoxylin and eosin (H&E) staining, and detection of isoform expression by Western blotting and immunohistochemistry (IHC), can then be carried out (Figure 2B). Critical steps, including the use of a high-salt protein extraction buffer, wet transfer, and BRD4 isoform-specific/Pan antibodies for detection of BRD4 protein isoforms (BRD4-L ~200 kDa and BRD4-S ~120 kDa), are highlighted in the accompanying Western blot procedure. When compared to semi-dry protein transfer, our protocol provides consistently better quality and reliability of high-molecular-weight protein transfer, with a much reduced background.
Materials and Reagents
Acetic Acid, Glacial, ACS grade (Fisher Scientific, catalog number: A38C-212)
Agarose Powder (Research Products International, catalog number: A20090-500.0)
Aprotinin (Fisher Scientific, catalog number: BP250310)
BRD4 Antibodies (in-house; described in Wu et al., 2006 and 2016)
α-Cre Antibody (Millipore, catalog number: 69050-3)
Direct PCR Lysis Reaction (Tail) Solution (Viagen, catalog number: 102-T)
NOTE: Do not use Direct PCR Lysis Reaction solution that has been open for more than one year. After this period, precipitates appear, and efficiency decreases significantly due to incomplete tail tissue lysis.
DNA Ladder, 1 kb Plus (Thermo Scientific, GeneRuler, catalog number: SM1333)
Dry Ice
Ear Tags (National Band and Tag Company, catalog number: 1005-1P)
ECL Detection Reagents:
West Femto Chemiluminescent Substrate (Thermo Scientific, SuperSignalTM, catalog number: PI34095)
ECL HRP Substrate (Advansta, WesternBright®, catalog number: K-12045)
Western Blotting Detection Reagents (Millipore-Sigma, ECLTM, catalog number: GERPN2209)
Ethidium Bromide, Molecular Biology grade (Sigma, catalog number: E7637-5G)
Ethyl Alcohol, 200 Proof (Pharmco, catalog number: 111000200)
Ethylenediaminetetraacetic Acid Sodium Salt (EDTA, Research Products International, catalog number: E57020-500.0)
Filter Paper:
Pure Cellulose Chromatography Paper (Fisher Scientific, FisherbrandTM, catalog number: 05-714-4)
Thick Blot Filter Paper, Precut, 7.5 × 10 cm (Bio-Rad, catalog number: 1703932)
Gel Loading Dye, Purple, 6× (New England BioLabs Inc., catalog number: B7024S)
Glycine (Fisher Scientific, catalog number: BP381-5)
Isopropyl Alcohol (2-Propanol, Fisher Scientific, catalog number: A416-4)
Labeling Tape, Self-Sticking (Fisher Scientific, catalog number: 1590110R)
Leupeptin (Fisher Scientific, catalog number: 50-114-6410)
Liquid Nitrogen
Methanol (Pharmco, catalog number: 339000000)
Mice (see Table 1)
Table 1. Mouse strains used in this protocol.
Microcentrifuge Tubes, 1.5 mL, Autoclaved (USA Scientific, catalog number: 1415-2500)
Nitrocellulose Membranes, Supported, 0.2 mm (Fisher Scientific, Cytiva AmershamTM ProtranTM, catalog number: 45-004-017)
NP-40 (Thomas Scientific, catalog number: C953H73)
PCR Primers (Sigma-Aldrich)
NOTE: See Table 2 for information on each primer sequence, annealing site, and product size.
Table 2. Primers used for mouse tail PCR reactions.
The databases, nucleotide sequences, and expected PCR sizes are indicated.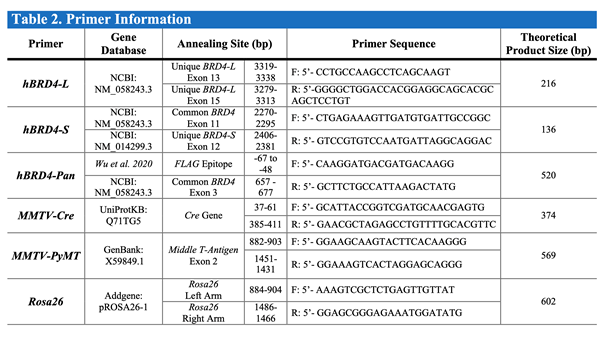
PCR Tubes, Dome Strip Caps, 0.2 mL, 8-Strip (Phenix Research Products, catalog number: MPX-445)
Pepstatin A (Fisher Scientific, catalog number: 501146412)
Phenylmethylsulfonyl Fluoride (PMSF, Sigma, catalog number: P7626)
Phosphatase Inhibitor Cocktail 2 (Sigma, catalog number: P5726)
Phosphate-Buffered Saline (PBS), pH 7.4Plastic Food Wrap (Kirkland Signature, catalog number: 208721)
Pre-Stained Protein Standards: Dual Color Standards (Bio-Rad, Precision Plus ProteinTM, catalog number: 1610374)
Duo Pre-Stained Protein Ladder (Li-Cor Biosciences Inc., Chameleon®, catalog number: 92860000)
Multicolor Broad Range Protein Ladder (ThermoFisher, SpectraTM, catalog number: 26634)
Protein Assay Dye Reagent Concentrate (Bio-Rad, catalog number: 500-0006)
Protein Gels, 4–20%, 15 Well, 15 µL (Bio-Rad, Mini-PROTEAN® TGX Stain-FreeTM, catalog number: 4568096)
Anti-Rabbit Antibody Conjugated with Horseradish Peroxidase (HRP) or Fluorescence:Goat Anti-Rabbit IgG Secondary Antibodies (Southern Biotechnology, catalog number: OB4050-05)
Anti-Rabbit IgG, 800 4× PEG Conjugate (Cell Signaling Technology, DyLightTM, catalog number: 5151)
Reactive Brown 10, Practical grade (Sigma-Aldrich, catalog number: R0385-25G)
Skimmed Milk Powder (Nestlé S.A., Carnation®)Sodium Chloride (NaCl, Fisher Scientific, catalog number: BP358-10)
Sodium Deoxycholate (Fisher Scientific, catalog number: BP349-100)
Sodium Dodecyl Sulfate (SDS, Fisher Scientific, catalog number: BP166-500)
Sodium Hydroxide Pellets, ACS grade (Fisher Scientific, catalog number: S318-3)
Tin Foil (Reynolds Wrap, catalog number: 2866C)
Tissue Homogenizing Tubes, 2 mL (Bertin Corp., CKMix, catalog number: P000918LYSK0A.0)
Tris Base (Research Products International, catalog number: T60040-5000.0)
α-β-Tubulin (H-235) Antibody (Santa Cruz Biotechnology, catalog number: sc-9104)
Tween 20 (Research Products International, catalog number: P20370)
Universal SYBR® Green Supermix, 2× (Bio-Rad, SsoAdvancedTM, catalog number: 1725274)
Water (Milli-Q®)Water, Nuclease-Free (Ambion, catalog number: AM9937)
X-Ray Film (Fisher Scientific, Cytiva AmershamTM HyperfilmTM ECL, catalog number: 45-001-508)
1× SDS-PAGE Buffer (see Recipes)
1× Wet Transfer Buffer (see Recipes)
20× TBS-T Buffer (see Recipes)
EDTA, 0.5 M, pH 8.0 (see Recipes)
Ethanol, 70% (see Recipes)
Ethidium Bromide Solution, 5 mg/mL (see Recipes)
mRIPA Buffer, 400 mM (see Recipes)
PCR Primer Working Solutions, 5 µM (see Recipes)
Proteinase K Solution, 20 mg/mL (see Recipes)
Tris-Acetate-EDTA Buffer, 1×, pH 8.3 (TAE, see Recipes)
Equipment
AC Imaging System (Ultra-Violet Products Ltd., BioSpectrumTM)
Analytical Balance (Mettler Toledo, catalog number: PG5002-S)
Centrifuge (Beckman Coulter, AllegraTM X-22R, catalog number: 392187)
NOTE: The S2096 rotor attachment is compatible with 0.2 mL PCR tubes.Centrifuge (Eppendorf, 5424, catalog number: EP-022620401)
NOTE: The FA-45-24-11 rotor attachment is compatible with the 1.5 mL microcentrifuge tubes.Ear-Tag Applicator, Stainless (Braintree Scientific, catalog number: EP1005S1)
Electrode Power Cables, Black & Red (Bio-Rad, Sub-CellTM GT)
Freezers, -20°C and -80°C Gel Combs, 15-Well, Fixed Height, 0.75 Thickness (Bio-Rad, Sub-CellTM GT, catalog number: 1704445)
Gel Eletrophoresis Tank (Bio-Rad, DNA Sub-CellTM)
Gel Imaging System for Fluorescence or Chemiluminescence: Odyssey Infrared Imaging System (Li-Cor Biosciences Inc., 9120)
Tabletop Film Processor (Konica Minolta, model: SRX-101A)
Gel Trey, UV-Transparent, 15 × 25 cm (Bio-Rad, Sub-CellTM GT, catalog number: 1704419)
Homogenizer (Bertin Corp., PreCellys® Evolution, catalog number: P000062-PEVO0-A)
Magnetic Stir Bar, Octagon (The Lab Depot, SpinBar®, catalog number: 58948-171-EA)
Magnetic Stirrer with Hot Plate (Fisher Scientific, IsoTempTM, catalog number: 11-100-49SH)
Microwave (Kenmore)
Mouse Dissection Tools
pH Meter (Fisher Scientific, FisherbrandTM Accumet Basic and BioBasicTM, catalog number: 13-636-AB15)
Power Supply (Bio-Rad, PowerPac 200)
Refrigerator, 4°CRoller (Bio-Rad, catalog number: 1651279)
Sonic Dismembrator (Fisher Scientific, FisherBrandTM, catalog number: FB120)
Tetra Vertical Electrophoresis Cell for Mini Precast Gels (Bio-Rad, Mini-PROTEAN®, catalog number: 1658005)
Thermal Cycler (Bio-Rad, T100TM Thermal Cycler)
Tweezers (Fisher Scientific, FisherbrandTM, catalog number: 12-000-123)
Vortexer (Scientific Industries Inc., Vortex-Genie 2 – 6560, catalog number: SI-0236)
Water Bath (Fisher Scientific, IsoTemp 220TM, catalog number: 15-462-20)
Wet Protein-Transfer Apparatus (Bio-Rad, Mini Trans-Blot Module, catalog number: 1703935)
Software
Adobe Photoshop 2020 (Version 21.2.4)
ImageJ (Version 1.53)
NOTE: Image J may be used instead of Adobe Photoshop to analyze the agarose gel and Western blot images.Li-Cor Image Studio (Version 5.2)
Microsoft Office Professional Plus (Excel)
Microsoft Office Professional Plus (PowerPoint)
UVP VisionWorksLS Image Acquisition and Analysis Software (Version 7.0.1)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Lewis, M. P., Wu, S. Y. and Chiang, C. M. (2022). Conditional Human BRD4 Knock-In Transgenic Mouse Genotyping and Protein Isoform Detection. Bio-protocol 12(7): e4374. DOI: 10.21769/BioProtoc.4374.
分类
癌症生物学 > 基因组不稳定性及突变 > 遗传学
癌症生物学 > 癌症生物化学 > 蛋白质
分子生物学 > DNA > 基因分型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link