- EN - English
- CN - 中文
Three-dimensional Models of the Nasopharynx for the Study of Epstein-Barr Virus Infection
研究EB病毒感染的鼻咽三维模型
发布: 2022年03月20日第12卷第6期 DOI: 10.21769/BioProtoc.4365 浏览次数: 4012
评审: Alka MehraSasidharan Swarnalatha LuckyYiqun Yu
Abstract
The ubiquitous and cancer-associated Epstein-Barr virus (EBV) is associated with nearly all cases of nasopharyngeal carcinoma (NPC). Nasopharyngeal tissue is comprised of both pseudostratified and stratified epithelium, which are modeled in three-dimensional (3-D) cell culture. The cellular origin of EBV-associated NPC is as yet unknown, but both latent and lytic infections are likely important for preneoplastic mechanisms and replenishing the compartmentalized viral reservoir. Conventional 2-D cultures of nasopharyngeal epithelial cells (as primary cells or immortalized cell lines) are difficult to infect with EBV and cannot mimic the tissue-specific biology of the airway epithelium, which can only be captured in 3-D models. We have shown that EBV can infect the pseudostratified epithelium in air-liquid interface (ALI) culture using primary conditionally reprogrammed cells (CRCs) derived from the nasopharynx. In this protocol, we provide a step-by-step guide for the (i) conditional reprogramming of primary nasopharyngeal cells, (ii) differentiation of CRCs into pseudostratified epithelium in ALI culture (known as pseudo-ALI), and (iii) EBV infection of pseudo-ALI cultures. Additionally, we show that nasopharyngeal CRCs can be grown as organotypic rafts and subjected to EBV infection. These nasopharyngeal-derived 3-D cell cultures can be used to study EBV latent and lytic infection in relation to cell type and donor variation, by immunostaining and single-cell RNA-sequencing methods (Ziegler et al., 2021). These methods are useful for studies of EBV molecular pathogenesis, and can overcome many of the limitations associated with conventional 2-D cell cultures.
Graphic abstract:
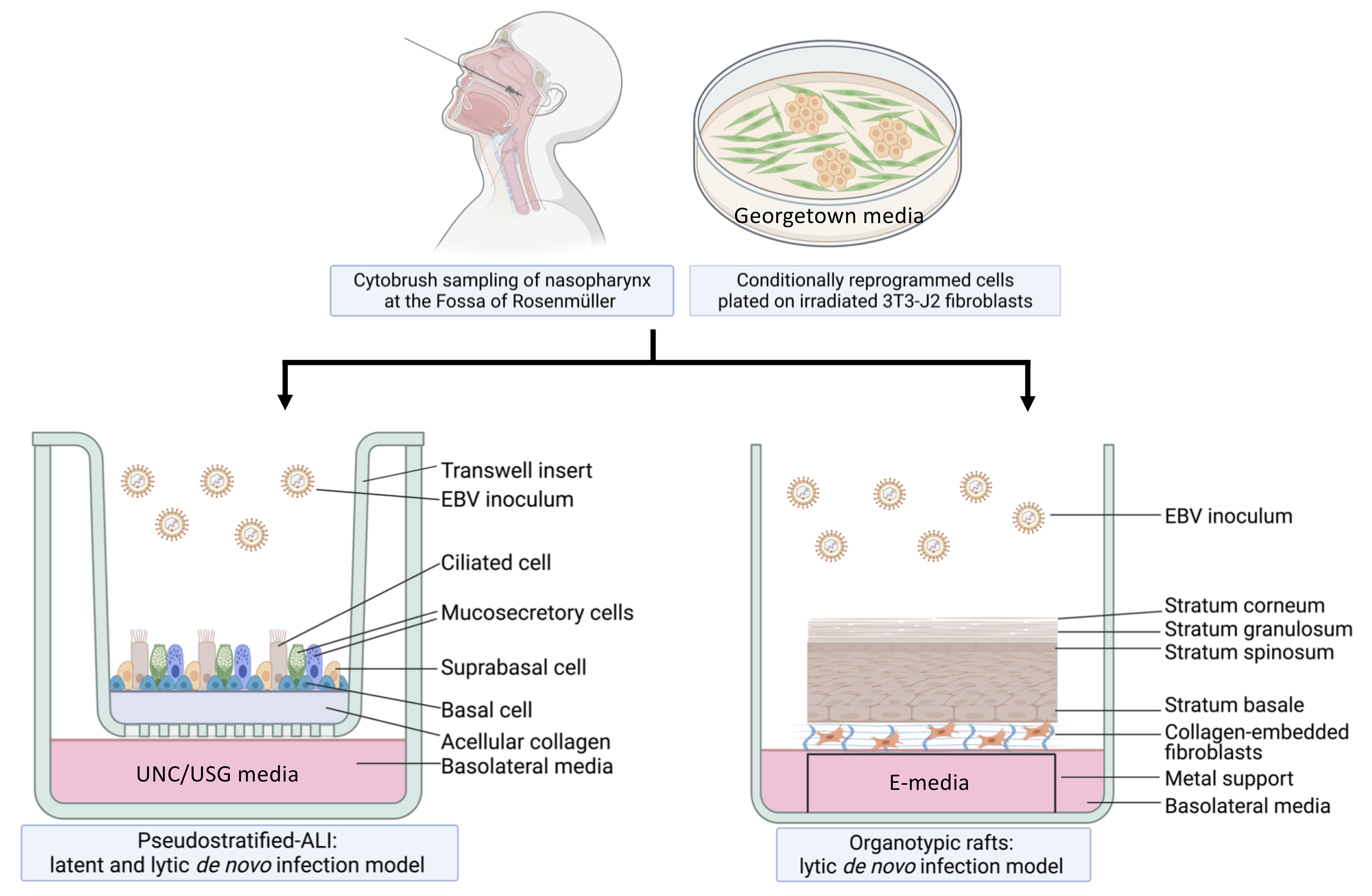
Workflow of nasopharyngeal-derived conditionally reprogrammed cells grown into pseudostratified-ALI and organotypic rafts in 3-D cell culture. Created with Biorender.com.
Background
EBV is a gammaherpes virus with both latent and lytic infection programs (Young et al., 2016). Latent infection is characteristic of nasopharyngeal carcinoma (NPC), but lytic infection and the production of progeny virus is important for replenishing the local viral reservoir (Tsao et al., 2012; Raab-Traub et al., 2015; Shair et al., 2018). More than 90% of people acquire EBV by adulthood and these chronic carriers continue to shed and transmit EBV in the nasal and oral cavities (Sitki-Green et al., 2003; Hadinoto et al., 2009). However, only a subset of individuals develops EBV-associated NPC, which occurs at high incidence in specific populations, such as South East Asians and Alaskan Innuits (Yu and Yuan, 2002). Both latent and lytic infection are thought to be important in the development of NPC, but this process has been difficult to study because of the rarity of EBV-infected nasopharyngeal cells in asymptomatic carriers, and the lack of physiologically relevant in vitro infection models (Caves et al., 2018; Shair, 2020). To address the question of which epithelial cell types in the nasopharynx are susceptible to EBV infection, and which cell types result in latent vs. lytic infection, we have built two distinct experimental models of the nasopharynx. The EBV infection program depends on epithelial differentiation, which is more accurately modeled in three-dimensional (3-D) culture. Thus, 3-D cell culture models overcome the shortcomings of previous EBV infection studies conducted in 2-D culture, which are limited to studies of EBV latency in outgrowing cell lines.
For the pseudostratified air-liquid interface model (pseudo-ALI model), a pseudostratified epithelium derived from nasopharyngeal conditionally reprogrammed cells (CRC) is exposed to de novo EBV infection by co-culture with anti-human IgG-reactivated EBV-positive B-cell inoculum (Maruo et al., 2001), or by cell-free virus infection. In the presence of Rho-associated protein kinase inhibitors (ROCKi), primary epithelial cells grown with feeder irradiated 3T3-J2 mouse fibroblasts are induced into a highly proliferative state by conditional reprogramming, which allows for propagation of primary cells while maintaining their differentiation potential (Chapman et al., 2010; Liu et al., 2012; Suprynowicz et al., 2012). Subsequently, UNC/USG (University of North Carolina/Ultroser G) specialized media is used to differentiate CRCs into mature pseudostratified airway epithelia in air-liquid interface cultures. Originally developed at the University of North Carolina (Fulcher and Randell, 2013), the UNC component has additives that induce the differentiation of human bronchial epithelial (HBE) and nasopharyngeal epithelial cells, which do not grow well in serum-containing media; thus, it’s necessary to use bovine pituitary extract (BPE) as a serum substitute. Other key constituents of the UNC media include a multitude of growth factors and trace elements that are essential for airway epithelial differentiation (Fulcher and Randell, 2013). These include retinoic acid, which promotes mucocilliary differentiation, and hydrocortisone, which promotes growth and epithelial sodium channel (ENaC) activity. The USG component is needed to promote robust electrophysiology (Gentzsch et al., 2017). The protocol we describe here can be used to differentiate both nasopharyngeal and bronchial epithelial cells (Fulcher and Randell, 2013; Serrano Castillo et al., 2019), but the EBV infection experiments we describe have only been attempted with nasopharyngeal cells.
Akin to EBV infection in organotypic rafts generated from primary oral keratinocytes (Temple et al., 2014, 2017), we generated nasopharyngeal-derived organotypic rafts to study EBV infection in the nasopharyngeal stratified epithelium, using CRCs which were exposed to reactivated EBV by the co-culture method. The principle of organotypic rafts is to generate multilayered stratified epithelia by differentiating keratinocytes at the air-liquid interface (Kopan et al., 1987) in defined E-media, which was originally described for the differentiation of oral (Temple et al., 2017) and cervical epithelial cells (Meyers, 1996). Here, we generated 3-D cell cultures using nasopharyngeal cells from donors undergoing skull base surgery (without any upper airway pathology), and from patients undergoing sinus surgery. CRCs can also be derived from the inferior turbinate from healthy persons without any co-pathology, provided such donors with no reason for surgery can be recruited. In sufficient quantity, the CRCs can be cryopreserved and thawed to generate both types of 3-D cell culture. A major advantage of using cryobanked cells is reproducibility and assessment of donor variability. These 3-D culture methods, when combined with EBV infection, are expected to reveal susceptible cell types in the upper airway, and uncover host determinants of EBV (latent and lytic) infection. A schematic of the workflow and measurable experimental outcomes are described in the Diagram below.
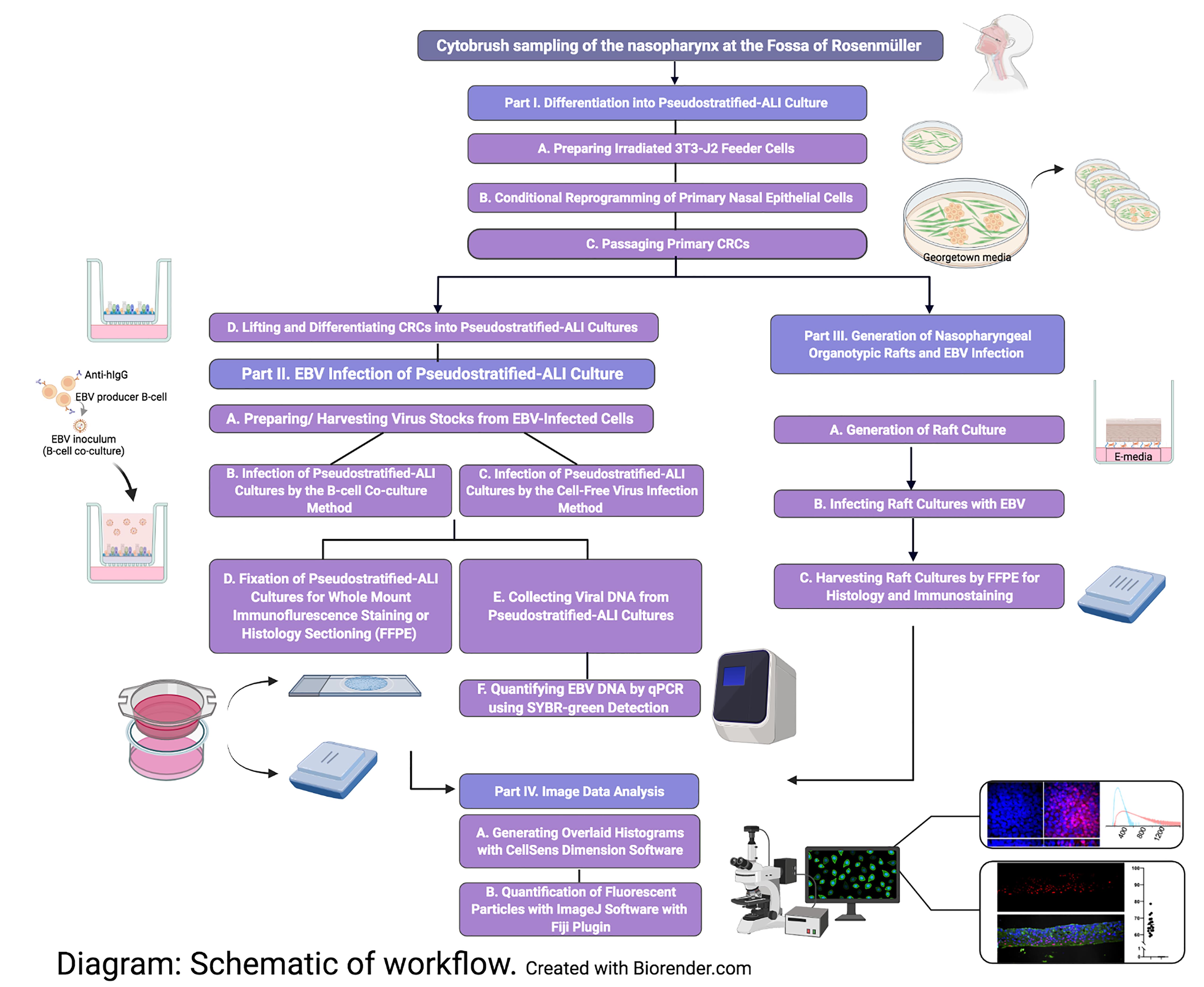
Part I: Differentiation into Pseudostratified-ALI Culture
Materials and Reagents
3T3-J2 Feeder Cells
Low passage 3T3-J2 cells (Kerafast, catalog number: EF3003)
3T3-J2 Growth Media
DMEM (Corning, catalog number: MT10013CV)
Bovine calf serum, iron-supplemented (GE Healthcare, catalog number: SH3007202)
0.05% Trypsin-EDTA (Corning, catalog number: MT25052CI)
DMSO (Fisher, catalog number: BP231-100)
100 mm tissue culture plates (Greiner, catalog number: 664160)
150 mm tissue culture plates (Greiner, catalog number: 639160)
Primary Nasopharyngeal Cells
Georgetown Media (see Recipes)
DMEM high glucose (Gibco, catalog number: 11965092)
Ham’s F-12 nutrient mix (Gibco, catalog number: 11765054)
100× Glutamine (Gibco, catalog number: 25030-081)
Fetal bovine serum (FBS) (Gibco, catalog number: 16140071)
Penicillin-streptomycin (Gibco, catalog number: 15140122)
Hydrocortisone (Sigma, catalog number: H0888)
EGF (Invitrogen, catalog number: PHG0311)
Insulin (Sigma, catalog number: I5500)
Amphotericin B, 250 µg/mL (Fisher, catalog number: BP264550)
Gentamicin, 10 mg/mL (Gibco, catalog number: 15710064)
Cholera toxin (Sigma, catalog number: C8052)
Y-27632 (Tocris, catalog number: 1254)
UNC/USG Media (see Recipes)
DMEM/F12 (Gibco, catalog number: 11320-033)
Penicillin-streptomycin (Gibco, catalog number: 15140122)
FeSO4·7H2O (Sigma, catalog number: F8633-250G)
MgCl2·6H2O (Sigma, catalog number: M2393-100G)
CaCl2·2H2O (Sigma, catalog number: C7902-500G)
ZnSO4·7H2O (Sigma, catalog number: Z0251-100G)
Selenium from Sodium selenite (Sigma, catalog number: S5261-100G)
Manganese from Manganese Chloride tetrahydrate (Sigma, catalog number: M8054-100G)
Silicon from Sodium Silicate nonahydrate (Sigma, catalog number: S4392-250G)
Molybdemun from Ammonium Molybdate tetrahydrate (Sigma, catalog number: M1019-100G)
Vanadium from Ammonium Metavanadate (Sigma, catalog number: 398128-50G)
Tin from Tin Chloride dihydrate (Sigma, catalog number: 243523-5G)
Transferrin (Sigma, catalog number: T8158-1G)
Hydrocortisone (Sigma, catalog number: H0888)
Insulin (Sigma, catalog number: I9278-5ML)
Epinephrine (Sigma, catalog number: E4250-10G)
T3 (Sigma, catalog number: T6397-100MG)
EGF (Corning, catalog number: 354052)
BSA (Sigma, catalog number: A9647-100G)
Retinoic Acid (Sigma, catalog number: R2625-50MG)
BPE (Pel-Freez, catalog number: 57136-3)
Phosphorylethanolamine (Sigma, catalog number: P-0503)
Ethanolamine (Sigma, catalog number: E9508-500ML)
Ultroser G (Pall, catalog number: 15950-017)
Amphotericin B, 250 µg/mL (Fisher, catalog number: BP264550)
0.25% Trypsin (HyClone, catalog number: SH3004201)
0.05% Trypsin/EDTA (Corning, catalog number: MT25052CI)
Hanks’ Balanced Salt Solution (HBSS) 1× with calcium and magnesium (Gibco, catalog number: 14025076)
Dulbecco’s Phosphate Buffered Saline (PBS) 1× without calcium and magnesium (Lonza, catalog number: 17512F)
Collagen IV (Sigma, catalog number: C7521)
Glacial Acetic Acid (Fisher, catalog number: BP2401-212)
Transwell Inserts, 24 well plate, polyester membrane, 0.4 µm pore size (Corning, catalog number: 3470)
Sterile Cytology Brush (Medical Packaging Corp, catalog number: CYB-1)
Equipment
Gamma-irradiator. We used the Gammacell 1000D model (Atomic Energy of Canada Ltd)
Humidified incubator at 37°C and 5% CO2, for cell culture
Biosafety cabinet Class II Type A2, for sterile cell culture work and biocontainment
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Ziegler, P., Reznik, A. S., Kitchloo, S. P., Wang, E., Lee, S. E., Green, A., Myerburg, M. M., Sample, C. E. and Shair, K. H. Y. (2022). Three-dimensional Models of the Nasopharynx for the Study of Epstein-Barr Virus Infection. Bio-protocol 12(6): e4365. DOI: 10.21769/BioProtoc.4365.
分类
微生物学 > 微生物-宿主相互作用 > 病毒
癌症生物学 > 瘤形成 > 离体组织培养模型
细胞生物学 > 细胞分离和培养 > 3D细胞培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link