- EN - English
- CN - 中文
Activity-based Protein Profiling of Serine Hydrolase Superfamily Enzymes
丝氨酸水解酶超家族酶的基于活性的蛋白质分析
发布: 2022年03月20日第12卷第6期 DOI: 10.21769/BioProtoc.4356 浏览次数: 2735
评审: Agnieszka ZienkiewiczAntoni William JamesAnonymous reviewer(s)
Abstract
Activity-based protein profiling (ABPP) is a chemoproteomics platform to assess the functional state of enzymes in complex biological systems. Over the two decades, ABPP has emerged from a gel-based to gel-free platform, for in-depth proteome analysis with enhanced resolution, sensitivity for target detection, and discovery of small molecule inhibitors. The gel-free format of ABPP coupled with advanced mass spectrometry is highly sensitive and provides more comprehensive knowledge for the targeted enzyme family than the gel-based method. ABPP strategy is applied across microbe, plant, and animal models. It can be performed both in vitro and in vivo studies, and there is no limitation on sample origin. Here, we report an ultrasensitive, gel-free format of ABPP called active site peptide profiling. This protocol describes the identification of authentic functional proteins, by tagging their active sites in a native biological system. It is high throughput in nature and helps enrich even low abundance functional proteins. Since protein identification is virtually based on a single peptide, the identified peptide should be a unique peptide to identify its parent protein. It can be performed in a facile manner and offers to consolidate identification of protein targets as well as the site of probe modification. We have validated this approach using a fluorophosphonate (FP) serine hydrolase probe in the native proteome of the cereal crop Oryza sativa.
Graphic abstract:
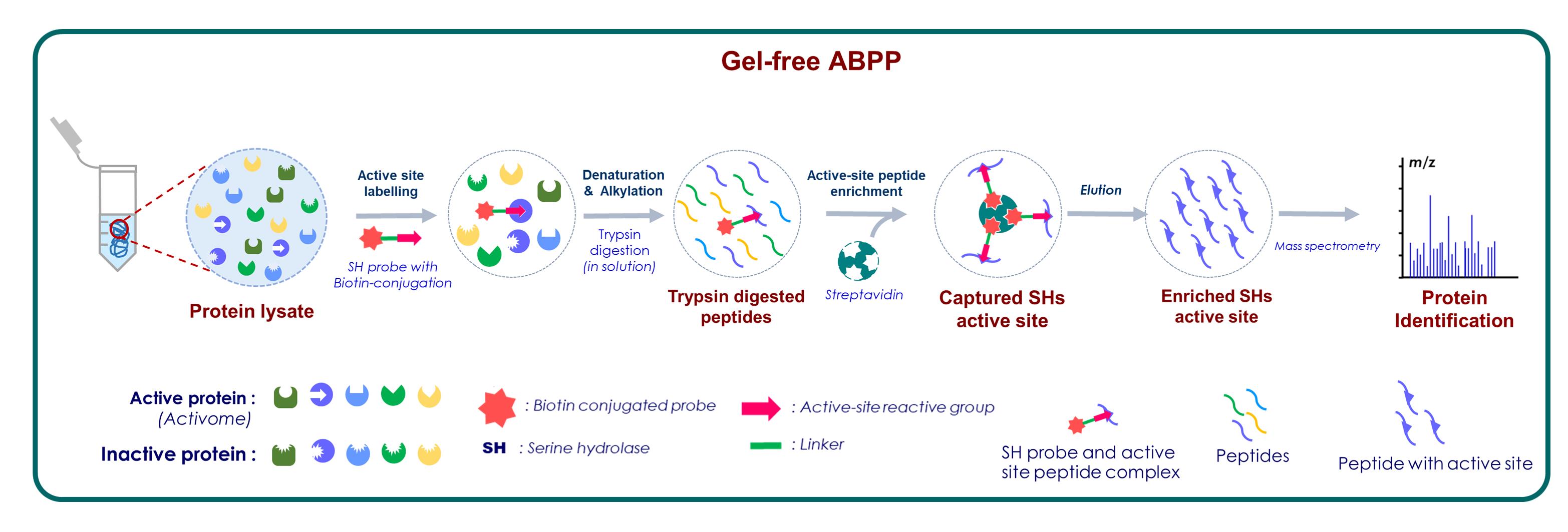
Serine hydrolase active site peptide profiling
Background
The advances in next-generation sequencing and long-read sequencing have opened the floodgate of many complex genomes. Inferring protein functions based on genome annotation may misannotate the catalytic properties to the uncharacterized genes, and it must be experimentally validated (Gerlt, 2016). Studying global analysis of gene transcription and translational changes by genomic and proteomic approaches gives only partial and indirect information about protein function. Further, protein activities are subjected to various post-translational modifications and regulations, which have pronounced effects on protein function (Bürkle, 2001). Therefore, conventional proteomic or genomic methods provide only an indirect assessment of the functional state of the enzyme. The lack of effective small-molecule probes for functional characterization has hindered our understanding of a myriad of functions and regulation modes. Activity-based proteomics is an analytical platform ideally suited for the global profiling of protein function (Schmidinger et al., 2006). In this approach, the functional annotation of uncharacterized proteins can be done precisely in an activity-dependent manner. Activity-based protein profiling (ABPP), pioneered by Cravatt and Bogyo, emerged as an efficient chemical proteomic approach for the analysis of functional states of target proteins in native biological systems (Evans and Cravatt, 2006, Cravatt et al., 2008, Niphakis and Cravatt, 2014). The application of ABPP depends on the quality of the probe, a small molecule that covalently binds to the active site of the target protein. In principle, an ABPP probe contains three parts: i) the reactive group, that forms a covalent bond with a conserved active site nucleophile, ii) a linker region, that attaches the reactive group with a reporter tag, and iii) a reporter tag, that provides a handle to detect and measure the protein labelled by the reactive group.
Since its inception, the most mature format for the visualization and characterization of probe-labelled proteomes in ABPP experiments is the in-gel fluorescence scanning (IGFS) (Patricelli et al., 2001). The identity of the proteins detected in IGFS can be achieved by enrichment followed by classical in-gel trypsin digestion (Dolui and Vijayaraj, 2020a; Latha et al., 2021). For example, replacement of a fluorescence tag with desthiobiotin facilitates the enrichment of the labelled enzymes via streptavidin/avidin affinity purification and subsequent identification through the mass spectrometric platform (Cravatt et al., 2008). This gel-based ABPP method directly links the labelling patterns of in-gel fluorescence signals and their identities (Phillips and Bogyo, 2005). However, there are some limitations to the gel-based ABPP method. The major limitation is the poor resolution of proteins that co-migrate on the gel, and identifying low-abundance proteins is difficult. With the advancement in proteomic techniques, ABPP has also significantly progressed from a gel-based to a virtually gel-free mode (Park et al., 2012). Since the activity-based probe covalently binds to the active site of functional proteins, the identity of the protein with the precise active-site residue information can be determined.
In this study, we described a gel-free format of ABPP for serine hydrolases active-site peptide profiling, using an active-site targeted probe (Dolui and Vijayaraj, 2020a). It provides comprehensive information for the active serine hydrolases with specific active-site residues mapping. In this approach, the number of probe-labelled active site peptides (in the trypsin digest of a probe-labelled proteome) logically equals the number of labelled proteins present in the sample (Okerberg et al., 2005). This gel-free ABPP format offers many advantages. First, performing an affinity enrichment step before mass spectrometry analysis helps to identify a low copy number of target enzymes. Therefore, it is high throughput in nature. Second, every single unique peptide will be able to reveal the identity of the parent target proteins. Since this peptide carries active site information, it is advantageous, and this information can be utilized for designing inhibitors for those target proteins. However, one of the prerequisites of this method is the accessibility for the active sites of enzymes in a native biological system. Therefore, pre-clearing the sample is an inevitable step prior to active site peptide profiling. Hence, we have developed a single-step pre-clearing method for lipid-rich samples for serine hydrolase labelling (Dolui and Vijayaraj, 2020b). Finally, multiple controls along with unbiased selection criteria should be adopted, to rule out any background and nonspecific proteins.
Materials and Reagents
1.7 mL microtubes (Axygen, catalog number: GEN-MT-175-C)
15 mL centrifuge tubes (Axygen, catalog number: GEN-CT-15 mL)
Gloves (Tarsons, catalog number: 370110)
Pipette tips (Axygen, catalog numbers: T-300 [10 µL], T-200-Y [200 µL], T-1000-B [1,000 µL])
Lab Apron (Medifit)
Zeba spin desalting columns, 7K MWCO, 5 mL (Thermo Scientific, catalog number: 89892)
ActiveX Desthiobiotin-FP Serine Hydrolase (Thermo Scientific, catalog number: 88317)
High capacity streptavidin agarose (Thermo Scientific, catalog number: 20357)
Dimethyl Sulphoxide, DMSO (Sigma, catalog number: D2650)
Dithiothreitol, DTT (Sigma, catalog number: D9779)
Iodoacetamide (Sigma, catalog number: I6125)
MS grade trypsin from porcine pancreas (Sigma, catalog number: T6567)
Trifluoroacetic acid,TFA (Sigma, catalog number: T6508-10AMP-KC)
LC-MS grade water (J.T. Baker, catalog number: 39253)
LC-MS grade acetonitrile, ACN (J.T. Baker, catalog number: 9829-03)
Urea (MP Biomedicals, catalog number: 194857)
Phosphate-buffered saline (PBS, pH 7.4)
NP-40 (Sigma, catalog number: NP40S)
Ethylenediaminetetraacetic acid, EDTA (MP Biomedicals, catalog number: 194822)
Tris (Himedia, catalog number: MB029-500G)
Sodium chloride, NaCl (Sigma, catalog number: S3014-500G)
Protein extraction buffer (see Recipes)
Assay buffer (see Recipes)
Washing buffer (see Recipes)
Elution buffer (see Recipes)
Solvent A (see Recipes)
Solvent B (see Recipes)
Equipment
-80°C freezer (New Brunswick, Ultra-low temperature freezer, model-U410), for long term storage of the probe
-20°C freezer (ARCTIKO)
Single-channel pipette (Eppendorf, model: Research Plus, catalog numbers: K31824D [10 µL capacity], L24004D [100 µL capacity], L25129D [1,000 µL capacity])
Ice flaking machine (Model-CB 246 HC)
Ice bucket
UV-Spectrophotometer (Jasco, V-630Bio) for protein quantification
ROTOSPINTM – End to end Test Tube Rotator (Tarsons, model: 3071)
Water bath (Grant, SAP5, SL. no. UD1541001)
AccuBlock digital dry bath (Labnet, model: D1100)
Centrifuge (Eppendorf, model: 5418)
Centrifuge (Eppendorf, model: 5810R) with 15 mL adapter
Ultracentrifuge (Beckman Coulter, model: Optima XPN-100)
Shaker (New Brunswick Scientific, SL. No. SE24FG001727)
Concentrator plus (Eppendorf, Code: E5305000363)
Vortex mixer (Velp Scientifica, Code No. F202A0176)
Weighing balance (Sartorius, model: CP423S)
Thermo Scientific LTQ or LTQ Orbitrap XL Mass Spectrometer (for analysis)
Pre-column (75 µm × 2 cm; Nanoviper C18, 3 µm; 100Å)
Liquid chromatography analytical column (RSLC C18 3 µm; 50 cm × 75 µm; 100Å)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Dolui, A. K., Latha, M. and Vijayaraj, P. (2022). Activity-based Protein Profiling of Serine Hydrolase Superfamily Enzymes. Bio-protocol 12(6): e4356. DOI: 10.21769/BioProtoc.4356.
- Dolui, A. K. and Vijayaraj, P. (2020a). Functional Omics Identifies Serine Hydrolases That Mobilize Storage Lipids during Rice Seed Germination. Plant Physiol 184(2): 693-708.
分类
植物科学 > 植物生物化学 > 蛋白质
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link