- EN - English
- CN - 中文
CD8 T Cell Virus Inhibition Assay Protocol
CD8 T 细胞病毒抑制实验方法
(*contributed equally to this work) 发布: 2022年03月20日第12卷第6期 DOI: 10.21769/BioProtoc.4354 浏览次数: 3538
评审: Alessandro DidonnaDhiman Sankar PalAnonymous reviewer(s)

相关实验方案
基于 T 细胞的平台,用于对肿瘤浸润 T 细胞的单细胞 RNA 测序数据集中鉴定的 T 细胞受体进行功能筛选
Aaron Rodriguez Ehrenfried [...] Rienk Offringa
2024年04月20日 6387 阅读
诱导型HIV-1库削减检测(HIVRRA):用于评估外周血单个核细胞中HIV-1潜伏库清除策略毒性与效力的快速敏感方法
Jade Jansen [...] Neeltje A. Kootstra
2025年07月20日 2457 阅读

研究免疫调控血管功能的新实验方法:小鼠主动脉与T淋巴细胞或巨噬细胞的共培养
Taylor C. Kress [...] Eric J. Belin de Chantemèle
2025年09月05日 3542 阅读
Abstract
The human immunodeficiency virus (HIV)-1 viral inhibition assay (VIA) measures CD8+ T cell-mediated inhibition of HIV replication in CD4+ T cells and is increasingly used for clinical testing of HIV vaccines and immunotherapies. Different VIAs that differ in length of CD8:CD4 T cell culture periods (6–13 days), purity of CD4 cultures [isolated CD4+ T cells or CD8+ depleted peripheral blood mononuclear cells (PBMCs)], HIV strains (laboratory strains, isolates, reporter viruses) and read-outs of virus inhibition (p24 ELISA, intracellular measurement of p24, luciferase reporter expression, and viral gag RNA) have been reported.
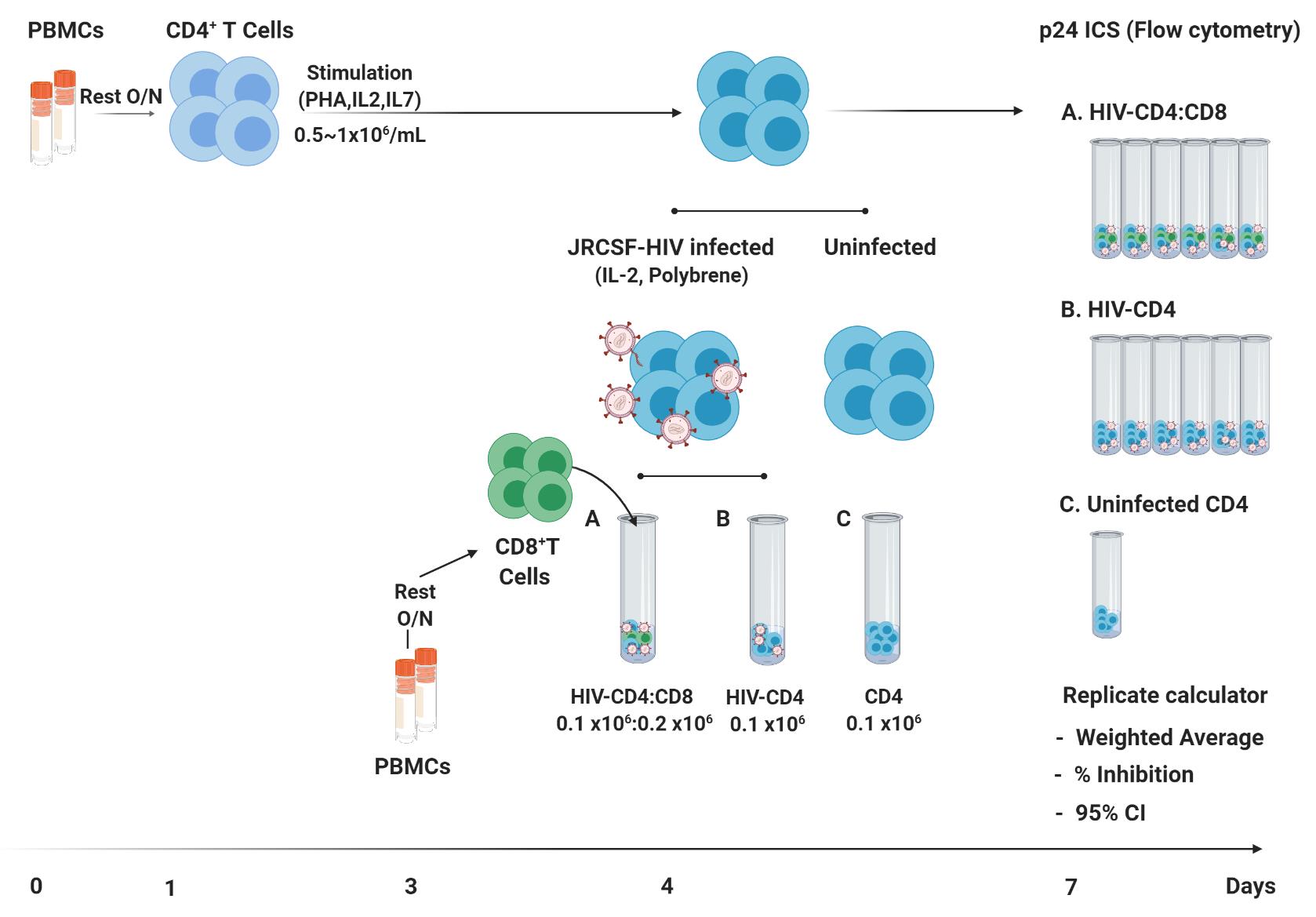
Here, we describe multiple modifications to a 7-day VIA protocol, the most impactful being the introduction of independent replicate cultures for both HIV infected-CD4 (HIV-CD4) and HIV-CD4:CD8 T cell cultures. Virus inhibition was quantified using a ratio of weighted averages of p24+ cells in replicate cultures and the corresponding 95% confidence intervals. We identify methodological and analysis changes that could be incorporated into other protocols to improve assay reproducibility. We found that in people living with HIV (PLWH) on antiretroviral therapy (ART), CD8 T cell virus inhibition was largely stable over time, supporting the use of this assay and/or analysis methods to examine therapeutic interventions.
Graphic abstract:
Background
A CD8+ T cell virus inhibition assay (VIA) measures the in vitro ability of CD8+ T cells to inhibit human immunodeficiency virus (HIV)-1 replication in autologous CD4+ T cells. This assay captures the full range of CD8+ T cell anti-viral activity in response to in vitro antigen presentation of virus-derived peptides by infected CD4 T cells. VIAs are increasingly used in clinical studies but mostly as an exploratory assay, because the complex co-culture of the VIA has substantial assay variability and a limited dynamic range. Different VIAs have been reported (Yang, O. O. et al., 1997; Sáez-Cirión et al., 2009, 2010; Spentzou et al., 2010; Julg et al., 2010; Freel et al., 2011; Yang, H. et al., 2012, 2013; Naarding et al., 2014; Slichter et al., 2014; Hancock et al., 2015) that differ in length of CD8:CD4 T cell culture periods (6–13 days), purity of CD4 cultures (isolated CD4+ T cells or CD8+ depleted PBMCs), HIV strains (laboratory strains, isolates, reporter viruses), and read-outs of virus inhibition (p24 ELISA, intracellular measurement of p24, luciferase reporter expression, and viral gag RNA). Here, we describe multiple modifications to a 7-day VIA protocol, the most impactful being the introduction of independent replicate cultures for both HIV infected-CD4 (HIV-CD4) and HIV-CD4:CD8 T cell cultures. Virus inhibition was quantified using a ratio of weighted averages of p24+ cells in replicate cultures and the corresponding 95% confidence intervals (CI). We found that in people living with HIV (PLWH) on antiretroviral therapy (ART), CD8+ T cell virus inhibition was largely stable over time, supporting the use of this assay and/or analysis methods to examine therapeutic interventions.
Materials and Reagents
FalconTM Round-Bottom Polystyrene Test Tubes (FalconTM, catalog number: 352054)
Falcon 50 mL Conical Centrifuge Tubes (Corning, catalog number: 352070)
Falcon 15 mL Conical Centrifuge Tubes (FalconTM, catalog number: 352097)
Phytohaemagglutinin (PHA) (Sigma-Aldrich, catalog number: L8902-5MG)
Benzonase® (Sigma-Aldrich, catalog number: NucleaseE1014)
CD4+ T Cell Isolation Kit (MACS, Miltenyi-Biotec, catalog number: 130-096-533)
HIV-1 JR-CSF Infectious Molecular Clone (pYK-JRCSF) NIH reagent program
IL-2 (Prometheus Proleukin, Aldesleukin, catalog number: 407682M)
IL-7 (PeproTech, catalog number: 200-07-10UG)
Polybrene (Santa Cruz Biotechnology, catalog number: NC9840454)
RPMI (Corning®, catalog number: 10-040-CV)
FBS (VWR Life Science, catalog number: 97068-091)
L-Glutamine (CorningTM, catalog number: 25005CI)
Sodium Pyruvate (CorningTM, catalog number: 25-000-CIR)
Penicillin-streptomycin (GibcoTM, catalog number: 15070063)
HEPES (CorningTM, catalog number: 25-060-CI)
PBS (CorningTM, catalog number: 21-030-CM)
BSA (Sigma, catalog number: A8412)
EDTA (CorningTM, catalog number: 46-034-CI)
Lysolecithin (Sigma, catalog number: L4129-25MG)
Paraformaldehyde (PFA) (Santa Cruz Biotechnology, catalog number: sc-281692)
Methanol (Sigma, catalog number: 34860-1L-R)
Nonidet P-40 (Biotang Inc, catalog number: BTBB914)
Zombie NIR Fixable Viability Kit (Biolegend, catalog number: 423106)
p24-FITCKC57-FITC (catalog number: 6604665, Beckman)
CD3-BV421 (BD Biosciences, catalog number: BDB562426)
CD4-AF488 (Biolegend, catalog number: 317434)
CD8-BV510 (Biolegend, catalog number: 344732)
Anti-mouse Ig, κ compensation beads (BDTM, catalog number: 552843)
PBMCs: Isolated from PLWH receiving antiretroviral therapy (HIVART) and seronegative individuals (healthy donor (HD))
R-10+ (see Recipes)
R-20+ (see Recipes)
Cell isolation buffer (see Recipes)
50% Methanol/PBS (see Recipes)
0.1% NP-40/PBS (see Recipes)
Equipment
Centrifuges (Thermo Scientific, ST40R TX-1000, catalog number: 50144036)
Muse Cell Analyzer (EMD Millipore Corporation, catalog number: 0500-3115)
Incubator (Thermo Scientific, catalog number: 190408340396)
MACS magnet (Miltenyi Biotech, OctoMACS Separator, catalog number: 130-042-108)
Flow Cytometer (BD LSRFortessa)
FINNPIPETTE F1 GLP (Thermo Scientific, catalog number: 4700850N)
Software
Excel (Microsoft)
Flow Jo (Flow Jo, LLC/ FlowJo_v10.8.0, https://www.flowjo.com/)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Xu, Y., Weideman, A. M., Abad-Fernandez, M., Mollan, K. R., Kallon, S., Samir, S., Warren, J. A., Clutton, G., Roan, N., Adimora, A. A., Archin, N., Kuruc, J., Gay, C., Hudgens, M. G. and Goonetilleke, N. (2022). CD8 T Cell Virus Inhibition Assay Protocol. Bio-protocol 12(6): e4354. DOI: 10.21769/BioProtoc.4354.
分类
免疫学 > 免疫细胞功能 > 淋巴细胞
免疫学 > 免疫细胞染色 > 流式细胞术
细胞生物学 > 基于细胞的分析方法 > 病毒性感染
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









