- EN - English
- CN - 中文
A Novel Standardized Peripheral Nerve Transection Method and a Novel Digital Pressure Sensor Device Construction for Peripheral Nerve Crush Injury
外周神经挤压伤的一种新型标准化外周神经横切方法及新型数字压力传感器的构建
发布: 2022年03月05日第12卷第5期 DOI: 10.21769/BioProtoc.4350 浏览次数: 3026
评审: Alessandro DidonnaHélène LégerAnonymous reviewer(s)
Abstract
Peripheral nerve injury (PNI) is common in all walks of life, and the most common PNIs are nerve crush and nerve transection. While optimal functional recovery after crush injury occurs over weeks, functional recovery after nerve transection with microsurgical repair and grafting is poor, and associated with permanent disability. The gold-standard treatment for nerve transection injury is microsurgical tensionless end-to-end suture repair. Since it is unethical to do experimental PNI studies in humans, it is therefore indispensable to have a simple, reliable, and reproducible pre-clinical animal model for successful evaluation of the efficacy of a novel treatment strategy. The objective of this article is two-fold: (A) To present a novel standardized peripheral nerve transection method in mice, using fibrin glue for modeling peripheral nerve transection injury, with reproducible gap distance between the severed nerve ends, and (B) to document the step-wise description of constructing a pressure sensor device for crush injury pressure measurements. We have successfully established a novel nerve transection model in mice using fibrin glue, and demonstrated that this transection method decreases surgical difficulties and variability by avoiding microsurgical manipulations on the nerve, ensuring the reproducibility and reliability of this animal model. Although it is quite impossible to exactly mimic the pathophysiological changes seen in nerve transection with sutures, we hope that the close resemblance of our novel pre-clinical model with gold-standard suturing can be easily reproduced by any lab, and that the data generated by this method significantly contributes to better understanding of nerve pathophysiology, molecular mechanisms of nerve regeneration, and the development of novel strategies for optimal functional recovery. In case of peripheral nerve crush injury, current methods rely on inter-device and operator precision to limit the variation with applied pressure. While the inability to accurately quantify the crush pressure may result in reduced reproducibility between animals and studies, there is no documentation of a pressure monitoring device that can be readily used for real-time pressure measurements. To address this deficit, we constructed a novel portable device comprised of an Arduino UNO microcontroller board and force sensitive resistor (FSR) capable of reporting the real-time pressure applied to a nerve. This novel digital pressure sensor device is cheap, easy to construct and assemble, and we believe that this device will be useful for any lab performing nerve crush injury in rodents.
Keywords: Peripheral nerve injury (周围神经损伤)Background
Peripheral nerve injury (PNI) represents a major health problem that often leads to significant functional impairment and permanent disability, resulting in annual costs of billions of dollars (Noble et al., 1998; Robinson, 2000; Foster et al., 2019; Karsy et al., 2019). PNI can range from simple compression to injuries with an intervening gap, where the nerve is completely separated (Campbell, 2008; Robinson, 2004; Caillaud et al., 2019). While optimal functional recovery after crush injury (axonotmesis, loss of axon continuity with preservation of nerve sheath) occurs over a period of 4–6 weeks (Hare et al., 1992; Moore et al., 2012; Caillaud et al., 2019), functional recovery after a severed nerve injury (neurotmesis, where the nerve itself is transected) with microsurgical repair and grafting is poor (Zochodne, 2012; Faroni et al., 2015), and full functional recovery is rare.
The available treatment for a completely severed nerve is end-to-end repair, nerve grafting, nerve conduits, or nerve transfer (Siemionow and Brzezicki, 2009; Isaacs, 2013). Poor functional recovery after these highly advanced microsurgical and reconstructive techniques could be partly due to the failure of these techniques to address the complex cellular and molecular events associated with PNI and repair (Zochodne, 2012; Faroni et al., 2015). To improve functional results for patients with PNIs, it is important to investigate multiple critical factors that are intimately related to the success of nerve regeneration and target innervation. While experimental studies to uncover mechanistic insights of PNI are impossible and unethical in humans, we recently established a novel standardized peripheral nerve transection technique in mice, which we refer to as Stepwise Transection and fibrin Glue (STG) (Lee et al., 2020), to investigate the mechanisms underlying poor functional outcomes. In the STG method, the nerve is first incompletely cut to 80% of its width, to prevent gap formation. Then, fibrin glue is applied around the laceration site, and the remaining 20% of the nerve is completely transected before the complete clotting of the glue. The STG method demonstrated post-injury pathophysiological changes comparable with the gold-standard suture repair, in terms of nerve histology and functional recovery (Lee et al., 2020). The STG method decreased surgical difficulties and variability by avoiding microsurgical manipulations on the nerve, thus ensuring the reproducibility and reliability of this animal model. Our novel transection method has several advantages as an experimental model when compared with current gold standard end-to-end neurorrhaphy, including: (i) standardized gap distance without microsurgical suturing, (ii) simplicity and reproducibility with faster procedure time, and (iii) lack of confounding factors associated with multiple suturing sites.
The sciatic nerve crush injury in rodents is a century-old model, widely used to study peripheral nerve regeneration and the potential beneficial effects of novel therapeutic strategies. Although crush injury can recover well within weeks in animal models (Hare et al., 1992; Moore et al., 2012), severe crush injury in humans with neuroma formation requires surgical intervention (Tos et al., 2012; Caillaud et al., 2019). Different tools (jeweler’s forceps, forceps with custom jig, malleus nipper, or needle driver), forces (~5 MPa to ~190 MPa), and duration (3 sec to 10 min) are used to create crush injuries (Chen et al., 1992; Bridge et al., 1994; Alant et al., 2012; Savastano et al., 2014; Tseng et al., 2016; Geary et al., 2017; Yue et al., 2019; Wandling et al., 2021), and there are concerns regarding the severity of crush injury pressure and the reproducibility of the method used. There have been limited attempts to quantify the pressure applied during crush injury in rodents, using a thin cell load for digital pressure display (Alant et al., 2012; Chen et al., 1992), and pressure-sensitive films for later analysis (Geary et al., 2017). However, there is no documentation of a pressure monitoring device that can be readily used for real-time pressure measurements. Importantly, operator precision and inter-device reliability may limit the standardization of the pressure applied and reproducibly of the findings. To address this gap, we constructed a novel portable device comprised of an Arduino Uno microcontroller board and force sensitive resistor (Figure 1), capable of reporting the real-time pressure applied to a nerve during crush injury (Wandling et al., 2021). We believe that the ability to deliver a more precise and quantifiable crush injury pressure using our novel device will increase standardization of crush injury and its reliability, leading to increased reproducibility of findings in the literature.
The objective of this article is two-fold: (A) To present a novel standardized peripheral nerve transection method in mice, that uses fibrin glue for modeling peripheral nerve transection injury with a reproducible gap distance between the severed nerve ends (Figures 2–3), and (B) to document the step-wise description of constructing a pressure sensor device (Figures 4–17) for crush injury pressure measurements.
Materials and Reagents
For the Stepwise Transection with fibrin Glue
Protective gear (sterile surgical gloves, goggles, lab coat, mask, hair cap)
1 mL Sub-Q syringe (Beckton, Dickinson Company, catalog number: 309597) and 1 mL U-100 Insulin syringe (Beckton, Dickinson Company, catalog number: 329420)
3MTM Micropore Surgical Paper tape (Fisher Scientific,catalog number: 19-061655)
Sterile Cotton Tipped Applicator (Puritan Medical Products, catalog number: 25-806 2WC) and CurityTM Gauze Pads (Covidien LLC, catalog number: 3381)
Autoclaved draping sheets and autoclaved surgical instruments (listed in Equipment section, as shown in Figure 2)
Surgical staples (3M Precise Disposable Skin Stapler, DS-25; 3M Health Care, MN, USA) for wound closure
Clean animal cage with food and water bottle
Animal cage warming pad (Right Temp Jr., catalog number: RT-JR-15 Kent Scientific, CT, USA) or warming station (Premiere Slide Warmer, Model XH-2004, available at Amazon)
10-week-old male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME, USA) weighing 20–25 g
Ketamine (60 mg/kg) and Xylazine (4 mg/kg) mixture (supplied by animal care facility)
Lubricant Ophthalmic ointment (Sterile Artificial Tears, Akorn Animal Health, Lake Forest, IL, USA)
Alcohol Prep (saturated with 70% ethanol; WebcolTM Alcohol Prep, catalog number: 5110, Covidien LLC, MA, USA) and McKesson 10% povidone-iodine solution (McKesson Corporation, catalog number: 854301PA)
Fibrin glue, consisting of fibrinogen and thrombin (Fibrin Sealant TISSEEL; Baxter Healthcare Corporation, catalog number: 1504516)
Slow release buprenorphine injection (0.05 mg/kg) (supplied by animal care facility)
Arduino Uno R3 (Arduino)
Micro SD TF card adapter reader module 6 pin (HiLetgo)
Standard LCD 16x2 Character Display Module (ELEDIY)
Micro SD card 32 GB (Samsung)
Prototype PCB shield board for Arduino Uno R3 (CZH-LABS)
Force sensitive resistor (FSR) (Flexiforce, A201, 4N)
Single row male PCB pin connectors (Honbay)
Toggle on/off switch (Hillman; 427680)
10 KOhm potentiometer (ELEDIY)
10 KOhm resistor (Elegoo)
220 Ohm resistor (Elegoo)
9V battery clip with 2.1 mm × 5.5 mm male DC plug (Corpco)
9V battery (Elegoo)
20-gauge solid wire (Lowes)
22-gauge stranded wire (Lowes)
Electrical tape (Lowes)

Figure 1. Materials for pressure sensor construction.
Equipment
For the Stepwise Transection with fibrin Glue
Operating microscope (Precision Stereo Zoom Binocular Microscope, Model PZMIII, World Precision Instruments, FL, USA)
Small animal scale (OHAUS, Model CS200)
Mouse clippers (All-in-one trimmer, Philips Norelco, Multigroom 3000)
Fine scissors (Figure 2A; Miltex, catalog number: MH18-1476, Integra LifeSciences, NJ, USA)
Surgical scissors (Figure 2B; Tekno-Medical, catalog number: tK8272-4)
Dissecting forceps (Figure 2C; Integra LifeSciences, catalog number: MH17-301)
Jeweler’s forceps (Figure 2D; Integra LifeSciences, catalog number: MH17-305)
Microscopic scissors (Figure 2E; Miltex, catalog number: MH18-1620, Integra LifeSciences, NJ, USA)
(Optional) Small Alm self-retaining retractor (Figure 2F; Tekno-Medical, catalog number: tK17330-07)
Heating pad (Right Temp Jr., catalog number: RT-JR-15, Kent Scientific, CT, USA)
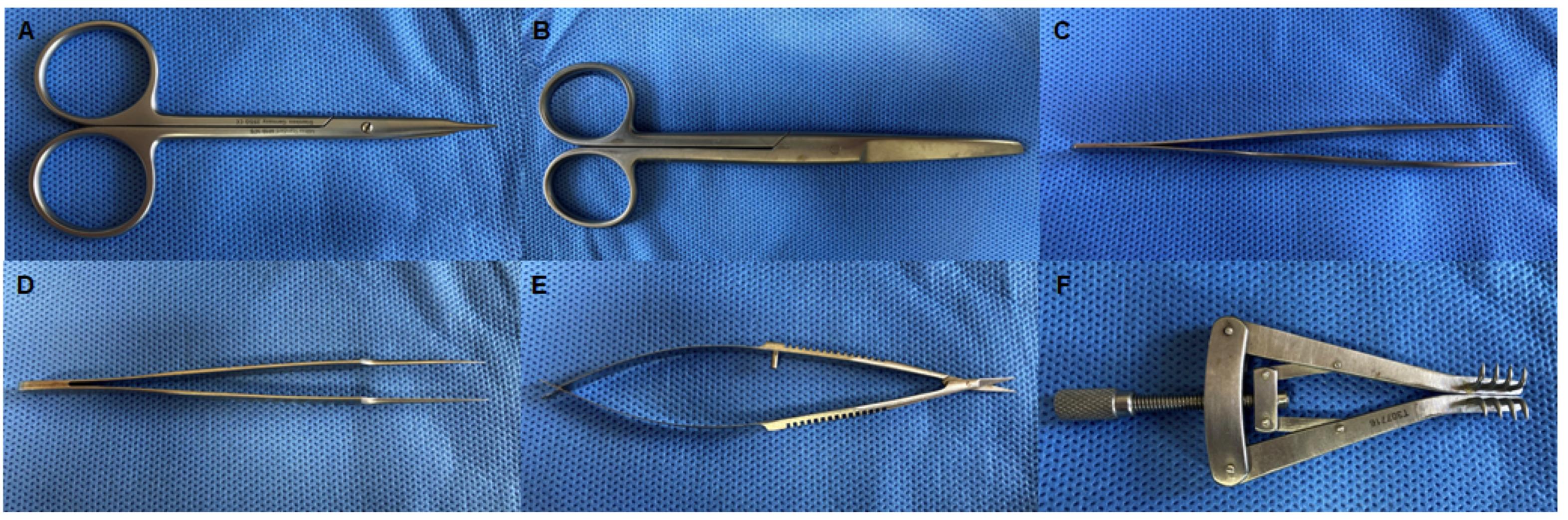
Figure 2. Surgical instruments for nerve transection.
Wire cutters (Lowes)
Needle nose pliers (Lowes)
Wire strippers (Lowes)
Soldering iron (Vastar)
Soldering wire (Tin/Lead; 60/40) (Flux 2.0%) (Vastar)
Flux soldering paste (Rubyfluid)
Software
For the Pressure Sensor
PrecisionPinch4.0 (Grant D Wandling, https://github.com/gwandling/PrecisionPinch)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Lee, J. I., Wandling, G. D., Talukder, M. A. H., Govindappa, P. K. and Elfar, J. C. (2022). A Novel Standardized Peripheral Nerve Transection Method and a Novel Digital Pressure Sensor Device Construction for Peripheral Nerve Crush Injury. Bio-protocol 12(5): e4350. DOI: 10.21769/BioProtoc.4350.
分类
神经科学 > 周围神经系统 > 坐骨神经
神经科学 > 神经系统疾病
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link