- EN - English
- CN - 中文
Labeling and Tracking Mitochondria with Photoactivation in Drosophila Embryos
果蝇胚胎中用光活化标记和跟踪线粒体
发布: 2022年03月05日第12卷第5期 DOI: 10.21769/BioProtoc.4347 浏览次数: 4321
评审: Gal HaimovichGülçin ÇAKAN AKDOĞANPradeep Kumar BhaskarAnonymous reviewer(s)
Abstract
Mitochondria are relatively small, fragmented, and abundant in the large embryos of Drosophila, Xenopus and zebrafish. It is essential to study their distribution and dynamics in these embryos to understand the mechanistic role of mitochondrial function in early morphogenesis events. Photoactivation of mitochondrially tagged GFP (mito-PA-GFP) is an attractive method to highlight a specific population of mitochondria in living embryos and track their distribution during development. Drosophila embryos contain large numbers of maternally inherited mitochondria, which distribute differently at specific stages of early embryogenesis. They are enriched basally in the syncytial division cycles and move apically during cellularization. Here, we outline a method for highlighting a population of mitochondria in discrete locations using mito-PA-GFP in the Drosophila blastoderm embryo, to follow their distribution across syncytial division cycles and cellularization. Photoactivation uses fluorophores, such as PA-GFP, that can change their fluorescence state upon exposure to ultraviolet light. This enables marking a precise population of fluorescently tagged molecules of organelles at selected regions, to visualize and systematically follow their dynamics and movements. Photoactivation followed by live imaging provides an effective way to pulse label a population of mitochondria and follow them through the dynamic morphogenetic events during Drosophila embryogenesis.
Keywords: Mitochondria (线粒体)Background
Drosophila melanogaster embryogenesis is a well-developed model system for studying mechanisms that drive plasma membrane shape changes during development. The Drosophila embryo is maternally loaded with machinery in the form of subcellular organelles, proteins, and mRNA, to drive the early stages of development. It is of interest to elucidate the function of organelle dynamics during early morphogenesis events in embryogenesis.
Drosophila embryogenesis begins as a syncytial cell, where nuclear cycles 1–13 occur in a common cytoplasm (Foe and Alberts, 1983). Nuclear division cycles 10–13 occur at the cortex. Complete cells that are epithelial in nature form in the interphase of syncytial division cycle 14. The plasma membrane extends around each nucleus and closes off at the bottom. Subcellular organelles, such as the endoplasmic reticulum (ER) and Golgi complex, are associated with each nuclear-cytoplasmic domain in the cortical syncytial division cycles (Frescas et al., 2006). Mitochondria are also present, interspersed within the ER and Golgi network (Chowdhary et al., 2017). Mitochondria are small, fragmented, and abundant in the syncytial blastoderm embryo (Chowdhary et al., 2017). Microtubule motors regulate their distribution in the syncytial division cycles and cellularization (Chowdhary et al., 2017 and 2020).
Genetically encoded fluorescently tagged transgenes distributed to the ER, Golgi complex, and mitochondria have helped ascertain their distribution in living embryos (Cox and Spradling, 2003; Frescas et al., 2006; Chowdhary et al., 2017). Diffusion dynamics of such fluorescently tagged molecules and organelles have been described using live imaging and photobleaching techniques, such as fluorescence recovery after photobleaching (FRAP) and fluorescence loss in photobleaching (FLIP). These techniques depend on bleaching of existing fluorescence and enable an analysis of local exchange rates of tagged molecules, at given regions of interest in cells (Frescas et al., 2006). Photoactivation involves the use of photo-convertible fluorophores that can switch their fluorescence properties upon photoactivation (Patterson, 2011). PA-GFP needs to be activated with a high-power light of approximately 400 nm wavelength, to show fluorescence at approximately 520 nm. Thus, photoactivation allows highlighting subcellular organelles during distinct stages of development in a relatively non-invasive manner, by activation of PA-GFP at a very specific location using the 400 nm light and tracking this marked population against the non-activated, non-fluorescent background (Patterson and Lippincott-Schwartz, 2002). Mito-PA-GFP has been used to study mitochondria dynamics in distinct stages of Drosophila oogenesis and embryogenesis (Chowdhary et al., 2017 and 2020; Lieber et al., 2019).
Cell shape change seen in cell migration, cytokinesis, and embryo development is accompanied by the mitochondrial movement to the location of shape transition (Denisenko et al., 2019). The presence of mitochondria locally in the Drosophila embryo is necessary for driving morphogenesis in embryo development (Chowdhary et al., 2017 and 2020). Visualization of mitochondria in Drosophila embryos using mito-GFP shows that they are abundant, and that their movements cannot be ascertained with precision due to their density (Chowdhary et al., 2017 and 2020). Highlighting mitochondria in specific regions via photoactivation, and then imaging their movement, is a powerful method to follow their dynamics during distinct morphogenetic transitions in embryo development. In this protocol, we describe photoactivation as a method for highlighting mitochondria in distinct regions in Drosophila embryo development. We provide a stepwise description of the photoactivation procedure followed by image analysis, to decipher the distribution of mitochondria during syncytial division cycles and cellularization. Using this protocol, we observed that photoactivation of mitochondria in mito-PA-GFP expressing embryos in one nuclear-cytoplasmic domain shows a lack of movement into adjacent domains (Chowdhary et al., 2017). Photoactivation of mitochondria basally during cellularization shows translocation to apical locations (Chowdhary et al., 2020).
Materials and Reagents
Drosophila melanogaster stocks - nanos-Gal4 [w1118; P{w[+mC]=GAL4::VP16-nos.UTR}CG6325MVD1, https://flybase.org/reports/FBti0012410 Bloomington Stock Center, #4937, https://bdsc.indiana.edu/ (Larkin et al., 2021)] and UASp-mito-PA-GFP (mito-PA-GFP) generated in the lab (Chowdhary et al., 2017). The mito-PA-GFP flies contain a transgene with the mitochondrial targeting sequence from human cytochrome oxidase VIII fused with PA-GFP.
Embryo collection cages (59-100, Flystuff.com)
Petri plates (60 mm, Tarsons, India)
Sucrose (non-molecular biology grade, local purchase)
Agar-agar (non-molecular biology grade, local purchase)
Sodium hypochlorite (Bleach) (Sigma, catalog number: 1056142500)
Cell strainer (Corning, catalog number: CLS431751)
Yeast (non-molecular biology grade, local purchase)
Malt (non-molecular biology grade, local purchase)
Cornflour (non-molecular biology grade, local purchase)
Propionic Acid (non-molecular biology grade, local purchase)
Orthophosphoric acid (non-molecular biology grade, local purchase)
Ethanol (non-molecular biology grade, local purchase)
NaCl (non-molecular biology grade, local purchase)
KCl (non-molecular biology grade, local purchase)
Na2HPO4 (non-molecular biology grade, local purchase)
KH2PO4 (non-molecular biology grade, local purchase)
2 chambered coverslip bottom dishes (Lab-Tek, chambered coverglass, no 1, Thermo 155380)
00 number paintbrush (Camlin, India)
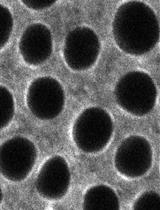
Cornmeal agar Drosophila medium in vials (see Recipes)
Sucrose agar plate (see Recipes)
Phosphate buffered saline (PBS) (see Recipes)
Note: During Drosophila oogenesis, mitochondria are transported from the nurse cells to the oocyte. To drive the expression of UASp-Mito-PA-GFP in the Drosophila ovarioles, nanos-Gal4 was used and, thus, tagged mitochondria were inherited into the embryos. nanos-Gal4 can be replaced by another tissue-specific driver of choice for expression of tagged mitochondria in other tissues.
Equipment
Incubator at 25°C for Drosophila stocks and crosses (Panasonic)
Stereo microscope (Olympus SZX10)
Confocal microscope (Carl Zeiss LSM 780)
Carl Zeiss stage stop incubator built-in with LSM 780 microscope
Software
ImageJ/Fiji - Bio-Formats plugin https://imagej.nih.gov/, https://imagej.net/software/fiji/ (Schindelin et al., 2012; Rueden et al., 2017)
Microsoft Excel https://www.microsoft.com/en-in/microsoft-365/excel
GraphPad Prism https://www.graphpad.com/scientific-software/prism/
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Chowdhary, S. and Rikhy, R. (2022). Labeling and Tracking Mitochondria with Photoactivation in Drosophila Embryos. Bio-protocol 12(5): e4347. DOI: 10.21769/BioProtoc.4347.
- Chowdhary, S., Madan, S., Tomer, D., Mavrakis, M. and Rikhy, R. (2020). Mitochondrial morphology and activity regulate furrow ingression and contractile ring dynamics in Drosophila cellularization. Mol Biol Cell 31(21): 2331-2347.
分类
发育生物学 > 形态建成 > 细胞结构
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link