- EN - English
- CN - 中文
High-speed Atomic Force Microscopy Observation of Internal Structure Movements in Living Mycoplasma
活体支原体内部结构运动的高速原子力显微镜观察
(*contributed equally to this work) 发布: 2022年03月05日第12卷第5期 DOI: 10.21769/BioProtoc.4344 浏览次数: 2996
评审: Kristin L. ShinglerRon Saar DoverAnonymous reviewer(s)
Abstract
Dozens of Mycoplasma species belonging to the class Mollicutes bind to solid surfaces through the organelle formed at a cell pole and glide in its direction by a unique mechanism. In Mycoplasma mobile, the fastest gliding species in Mycoplasma, the force for gliding is generated by ATP hydrolysis on an internal structure. However, the spatial and temporal behaviors of the internal structures in living cells were unclear. High-speed atomic force microscopy (HS-AFM) is a powerful method to monitor the dynamic behaviors of biomolecules and cells that can be captured while maintaining their active state in aqueous solution. In this protocol, we describe a method to detect their movements using HS-AFM. This protocol should be useful for the studies of many kinds of microorganisms.
Graphic abstract:
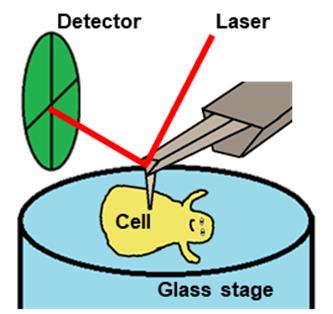
Scannnig Mycoplasma cell
Background
Class Mollicutes, a small group of bacteria featuring a single-layered cell membrane, has as many as three unique motility mechanisms different from other organisms. Recently, the isolated internal structure of M. mobile was shown to hydrolyze ATP with conformational changes, suggesting that the internal structure functions as a force generator for gliding (Miyata and Hamaguchi, 2016; Nishikawa et al., 2019). However, the spatial and temporal behaviors and movements of internal motors in living cells have not been examined.
High-speed atomic force microscopy (HS-AFM) is a powerful method to monitor the dynamic behaviors of biomolecules and cells that can be captured while maintaining their active state in aqueous solution (Ando, 2018). In recent years, this approach has been dramatically improved, and the behaviors of more and more proteins have been elucidated (Kodera et al., 2010 and 2021; Ando, 2018; Kodera and Ando, 2020). In addition, HS-AFM has been applied to understanding the structures in the cell wall surface (Yamashita et al., 2012) and below the cell membrane (Zhang et al., 2017).
Here, we provide a protocol detailing how to detect the movements of the internal structure in a living M. mobile cell, based on our recent study (Kobayashi et al., 2021).
Materials and Reagents
1.5 mL microtube (Greiner Bio-One, catalog number: 616201)
Glass substrate stage for HS-AFM (custom-made glass rod with 2 mm in diameter and 2 mm in height, Japan Cell)
Cantilever (Olympus, BLAC10DS-A2)
#3000 sand paper (Riken Corundum, Precision Polishing Film Sheet #3000, catalog number: 3-9013-06)
100 mL beaker
Kimwipes (Nippon Paper Crecia, catalog number: 62015)
Filter paper for liquid replacement on glass stage (ADVANTEC, catalog number: 01531090) (Cut into triangles as shown in Figure 2)
25 cm2 tissue culture flask (AS ONE, catalog number: 2-8589-01)
Micro pipette tip (Greiner Bio-One, catalog numbers: 740290 and 739285)
Acetone (FUJIFILM Wako Pure Chemical Corporation, catalog number: 012-00343)
Potassium hydroxide (KOH) (Nacalai Tesque, catalog number: 28616-45)
Ethanol (FUJIFILM Wako Pure Chemical Corporation, catalog number: 057-00456)
Fluorosurf (FluoroTechnology, catalog number: FG-3020C-20)
Colorless nail polish (Shiseido, MAQuillAGE (Top base coat))
Deionized water
3-aminopropyldiethoxymethylsilane (APTES) (Shin-Etsu Chemical, catalog number: KBE-903)
Glutaraldehyde (Sigma-Aldrich, catalog number: G5882)
Heart infusion broth (BD, catalog number: 238400)
Yeast extract (BD, catalog number: 212750)
10 N NaOH (Nacalai Tesque, catalog number: 31511-05)
Horse serum (Thermo Fisher Scientific, GibcoTM, catalog number: 16050122)
Amphotericin B (Sigma-Aldrich, catalog number: A2942)
Ampicillin Na (Nacalai Tesque, catalog number: 02739-32)
di-Sodium hydrogenphosphate (Nacalai Tesque, catalog number: 31801-05)
Sodium dihydrogenphosphate dihydrate (FUJIFILM Wako Pure Chemical Corporation, catalog number: 192-02815)
NaCl (Nacalai Tesque, catalog number: 31320-05)
Glucose (Nacalai Tesque, catalog number: 16806-25
Phosphate-buffered saline containing glucose (PBS/G) (see Recipes)
Aluotto medium (see Recipes)
A mutant strain (gli521[P476R]) isolated from M. mobile 163K strain (ATCC, catalog number: 43663) (Uenoyama and Miyata, 2005)
Equipment
Micro pipette (Gilson, catalog numbers: F123600, F123601, and F123602)
Centrifuge (Sigma Laborzentrifugen, model: Sigma 1-14)
25°C incubator (Tokyo Rikakikai, model: LTI-400E)
Heater (TAITEC CORPORATION, CTU-Neo)
Magnetic stirrer (AS ONE, model: CT-1AT)
HS-AFM (An apparatus with the same performance is commercially available from Research Institute of Biomolecule Metrology Co., Ltd., model: SS-NEX)
Autoclave (TOMY SEIKO, model: LSX-700)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Kobayashi, K., Kodera, N. and Miyata, M. (2022). High-speed Atomic Force Microscopy Observation of Internal Structure Movements in Living Mycoplasma . Bio-protocol 12(5): e4344. DOI: 10.21769/BioProtoc.4344.
分类
微生物学 > 微生物细胞生物学 > 细胞运动性
细胞生物学 > 细胞运动 > 细胞运动性
细胞生物学 > 细胞成像 > 原子力显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link