- EN - English
- CN - 中文
Pull-down of Biotinylated RNA and Associated Proteins
生物素化 RNA 和相关蛋白的下拉
发布: 2022年02月20日第12卷第4期 DOI: 10.21769/BioProtoc.4331 浏览次数: 8565
评审: Chiara AmbrogioKarem A CourtZheng Zachory WeiAnonymous reviewer(s)
Abstract
Mapping networks of RNA-protein interactions in cells is essential for understanding the inner workings of many biological processes, including RNA processing, trafficking, and translation. Current in vivo methods for studying protein-RNA interactions rely mostly on purification of poly(A) transcripts, which represent only ~2–3% of total RNAs (Figure 1). Alternate robust methods for tagging RNA molecules with an RNA aptamer (e.g., MS2-, U1A- and biotin-RNA aptamer) and capturing the RNA-protein complex by the respective aptamer-specific partner are not extensively studied. Here, we describe a protocol (Figure 2) in which a biotin-RNA aptamer, referred to as the RNA mimic of biotin (RMB), was conjugated separately to two small RNA secondary structures that contribute to trafficking and translating HAC1 mRNA in the budding yeast Saccharomyces cerevisiae. The RMB-tagged RNA was expressed in yeast cells from a constitutive promoter. The biotinylated RNA bound to proteins was pulled down from the cell lysate by streptavidin agarose beads. RNA was detected by RT-PCR (Figure 3) and associated proteins by mass spectrometry (Figure 4). Our findings show that an RNA aptamer tag to RNA molecule is an effective method to explore the functional roles of RNA-protein networks in vivo.
Keywords: RNA binding protein (RBP) (RNA结合蛋白(RBP))Background
RNA is a multifaced biomolecule with a wide range of biological functions. For example, messenger RNA (mRNA) carries the instructions for protein synthesis, ribosomal RNA (rRNA) provides the structural and enzymatic framework of the ribosome, and transfer RNA (tRNA) contains the anticodon of specific mRNA codons and delivers amino acids corresponding to the codon during translation. RNAs also play key regulatory partners for certain proteins involved in a wide variety of biological processes, including gene expression during growth, development, and cellular stress (Hudson and Ortlund, 2014). These RNA binding proteins (RBPs) contain one or more RNA-binding domains (RBD), which recognize and bind to specific sequences at their target RNA. Unraveling the specificity in RNA-protein recognition is therefore key to understanding the mechanisms by which cellular pathways are controlled and connected at the molecular level.
Several in vivo and in vitro strategies are being used to identify global RBPs, including a novel in vivo technique called RNA interactome capture (RIC) (Keene et al., 2006; Castello et al., 2013; Jazurek et al., 2016; Kastelic and Landthaler, 2017). Typically, RIC involves crosslinking proteins to mRNAs in vivo, immunoprecipitation (IP) of the mRNA-protein complex (CLIP) by using oligo(dT) magnetic beads, and identification of covalently linked proteins by mass spectrometric based methods and RNA fragments, by reverse transcriptase (RT) PCR and sequencing (Ramanathan et al., 2019) (Figure 1).

Figure 1. Schematic representation of the RNA interactome capture (RIC). Proteins are crosslinked to RNA in vivo by UV irradiation. Yellow light box: RNA binding proteins (RBPs) bound to poly(A) RNAs are shown by various colored shapes, and unbound proteins are shown by grey circles. Light green box: The RNA-protein complex is recovered from the unbound proteins by oligo-d(T) 25 beads. Proteins and RNA fragments are subjected to limited RNase treatment. Proteins are identified by quantitative mass spectrometry. RNA fragments occupied by RBP are subjected to sequencing to identify the RBP binding sites.
CLIP enables the identification of several RBPs with well-defined RNA binding domains, such as the RNA recognition motif (RRM), the zinc finger (Znf) domain, or the K-homology (KH) motif. Additionally, CLIP identifies novel RBPs that do not have a canonical RNA binding domain, but instead have intrinsically disordered regions (IDRs), such as S/R repeats, RG/RGG repeats, Q/N-rich stretches and short linear motifs (SLiMs) (Gerstberger et al., 2014, Hentze et al., 2018, Balcerak et al., 2019). These disordered regions in RBPs are often dynamic and flexible, with low-ordered secondary structures that enable them to bind to multiple RNA partners. The low-ordered structure transits to an ordered conformation upon binding to their partner RNA (Srivastava et al., 2021). Additionally, a single RBP can bind to several RNA targets (Muller-McNicoll and Neugebauer, 2013), thus necessitating the direct measurement of RNA binding by RBP to establish the functional interaction.
Many RBPs bind to the 5’- and/or 3’-unstranslated regions (UTR) of mRNAs and regulate their storage, stability, localization, and/or translational efficiency. Among RBPs that bind to the specific 3’-UTR sequence (5’-AUUUAU-3’), also known as the AU-rich element (ARE), are AUF1 (Brewer, 1991), tristetrapolin (Lai et al., 1999), and HuR (Fan and Steitz, 1998). These ARE-BPs are known to promote stabilization, degradation, or translatability of mRNAs (Otsuka et al., 2019); however, the molecular mechanisms that regulate their targets are still unknown.
Recent high-throughput sequencing technologies, including SHAPE-Seq (selective 2′-hydroxyl acylation analyzed by primer extension sequencing) and Frag-Seq (fragmentation sequencing), revealed that >90% of 5’- and 3’-UTR in mRNAs could form secondary structures, in both yeast (Kertesz et al., 2010) and human transcriptomes (Wan et al., 2014, Bevilacqua et al., 2016). Combined with CLIP and the parallel analysis of RNA structure (PARS), Groot et al. recently showed that RNA-protein interaction largely depends on their contextual structures (Sanchez de Groot et al., 2019). They also show that cellular proteins bind to single- and double-stranded RNA. To date, RBP2GO (https://rbp2go.dkfz.de) has collectively listed more than 22,000 RBPs in 13 organisms, including the budding yeast Saccharomyces cerevisiae. However, the specific RNA-protein interaction networks and physiological functions have not been fully assigned.
The CLIP method relies on oligo-dT beads, thus limiting it to poly(A) RNAs, which represent only ~2–3% of total RNAs (Djebali et al., 2012; Uszczynska-Ratajczak et al., 2018). For non-poly(A) RNAs, the potential solution is to tag them with an RNA aptamer and capture the RNA-protein complexes by the respective aptamer-specific protein. The commonly used RNA aptamers are 19-nucleotides MS2 RNA hairpin (5′-ACAUGAGGAU CACCCAUGU-3’) (Yoon et al., 2012), 15–19-nucleotides BoxB RNA hairpin (5′-NNGCCCTGAA GAAGGGCNN-3’) (Baron-Benhamou et al., 2004, Cocozaki et al., 2008), 29-nucleotides U1A RNA hairpin (5′-AGCUUAUCCA UUGCACUCCG GAUGAGCU-3′) (Katsamba et al., 2001), and 43-nucleotides biotin-RNA aptamer, referred to as the RNA mimic of biotin (RMB, 5′-ACCGACCAGA AUCAUGCAAG UGCGUAAGAU AGUCGCGGGC CGGG-3′) (Vasudevan and Steitz, 2007). The MS2 approach has been valuable in targeting and identifying the specific RNA-protein complex (Yoon et al., 2012), indicating that other tagging methods could also potentially be used. However, each of these RNA tagging methods has specific drawbacks, in that it can affect the function of the target RNA. Therefore, it is important to select a method based on the biological question being asked.
In this report, we elaborate a protocol in which an RMB was conjugated to two small RNA secondary structures that contribute to targeting and translating HAC1 mRNA (Ruegsegger et al., 2001) in the budding yeast S. cerevisiae. The first one is a 60-nucleotide RNA molecule representing the 3′-bipartite element (3′-BE) (Aragon et al., 2009), and the second one is a 40-nucleotide RNA molecule representing the 5’-UTR-intron RNA duplex (5’-RD) (Ruegsegger et al., 2001). Both 5’-RD-RMB and 3′-BE-RMB were expressed from a constitutive ADH1 promoter in yeast cells grown in the presence of 4-thiouracil (4-SU). Cells were harvested and irradiated with UV light. The 3′BE-RMB-protein and the 5’-RD-RMB-protein complexes were pulled down from the cellular lysate by streptavidin-agarose beads (Figure 2).

Figure 2. The workflow diagram of the RNA and protein pulldown and analysis. Yeast cells expressing 3’-BE-RMB or 5’-RD-RMB were grow till OD600 reached ~0.6. DTT or 4-thiouracil (4-SU) were added to the culture and the cells grown for an additional 3 hours. Cell were collected in a 50 mL Falcon tube, washed with 1× PBS, irradiated with UV light, and harvested. Cells were lysed and divided into two Eppendorf tubes. In both tubes, streptavidin agarose was added and mixed for 1 hour at 4°C. Tubes were centrifuged to separate pellet (P) and supernatant (S) fractions for subsequent RNA or protein analysis.
The RMB-tagged RNA was detected by RT-PCR (Figure 3). The covalently associated protein was visualized by Coomassie and Silver-staining methods (Figure 4) and identified by mass spectrometry (Ghosh et al., 2021). We also showed that an uncharacterized protein Pal2 specifically bound to the synthetic 3’-BE (Ghosh et al., 2021). Our findings indicate that an RNA tag to a small RNA molecule is an effective method to explore the functional roles of RNA-protein networks in vivo.
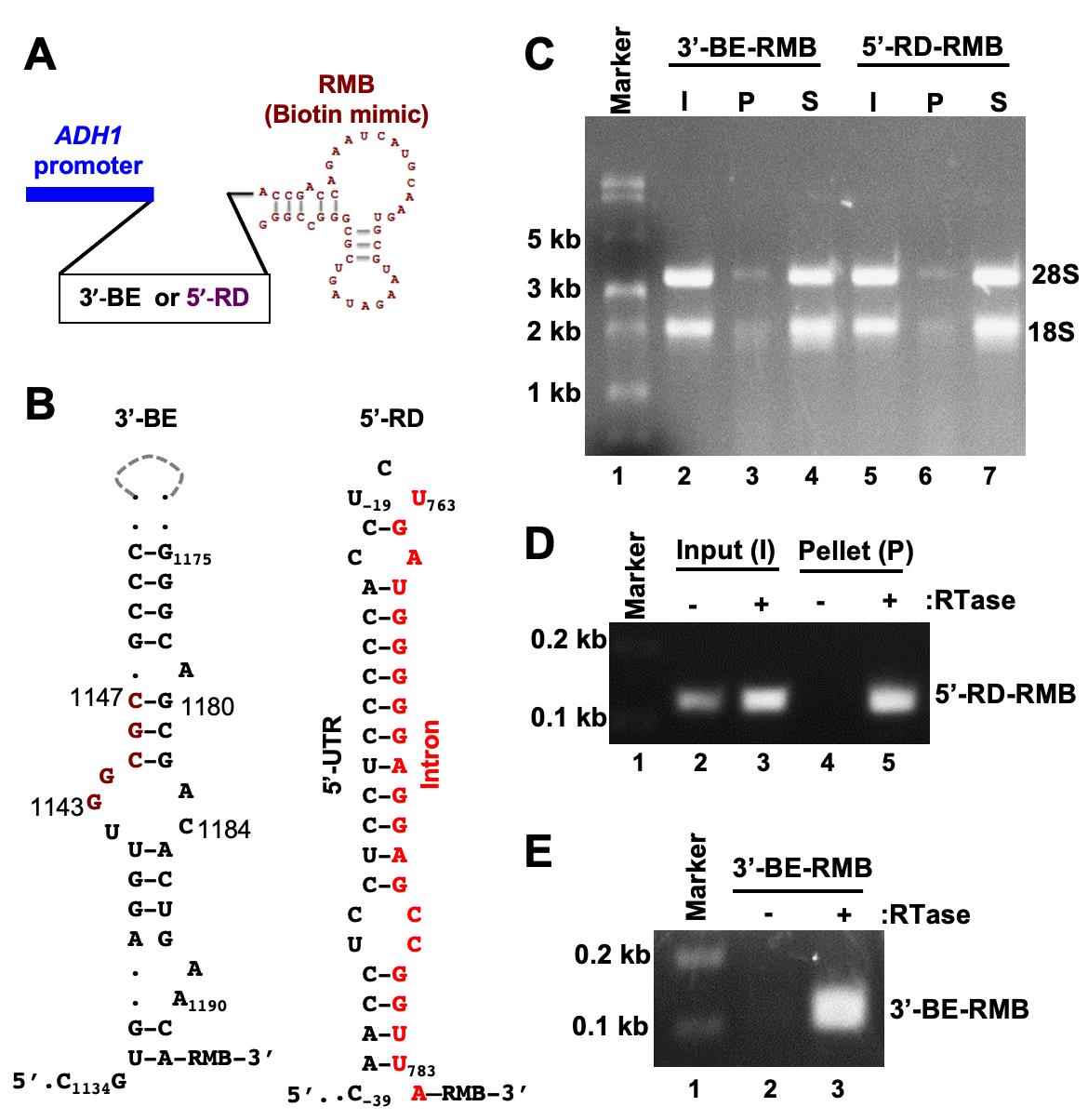
Figure 3. Pulldown of biotinylated RNA. A. The template DNA sequence of the 3’-BE- or 5’-RD RNA was placed under the control of the constitutive ADH1 promoter. The predicted structure of the RMB is shown. B. The predicted secondary structures of 3’-BE and 5’-RD RNAs are shown. The conserved RNA motif within the 3’-BE is shown in red. The 5’-RD consisting of 5’-UTR and intron of HAC1 mRNA, which are shown in black and red, respectively. The numbers indicate the nucleotide positions of HAC1 mRNA. C. Whole cell extract (WCE) from yeast cells expressing the biotinylated RNA (3’-BE-RMB or 5’-RD-RMB RNAs) was prepared and mixed with streptavidin agarose for 1 hour at 4°C. The mixture was centrifuged to separate the pellet (P) and supernatant (S) fractions. RNA was isolated using a Trizol method and equal amounts of RNA (500 ng) from the pellet and supernatant fractions were loaded onto a denaturing formaldehyde agarose gel, to monitor the integrity of RNA. As an input (I), 500 ng RNA from the WCE was also separated. D. To analyze 5’-RD-RMB mini-RNA expression, cDNA was synthesized from total RNA isolated from input (I) and pellet (P) fractions, using gene-specific primers in the presence and absence of reverse transcriptase (RTase). The cDNA was then used as a template to amplify the 5’-RD RNA. E. To analyze 5’-BE-RMB mini-RNA expression, cDNA was synthesized from total RNA isolated from the P fraction using gene-specifics primers in the presence and absence of reverse transcriptase (RTase). The cDNA was then used as a template to amplify the 5’-BE RNA.
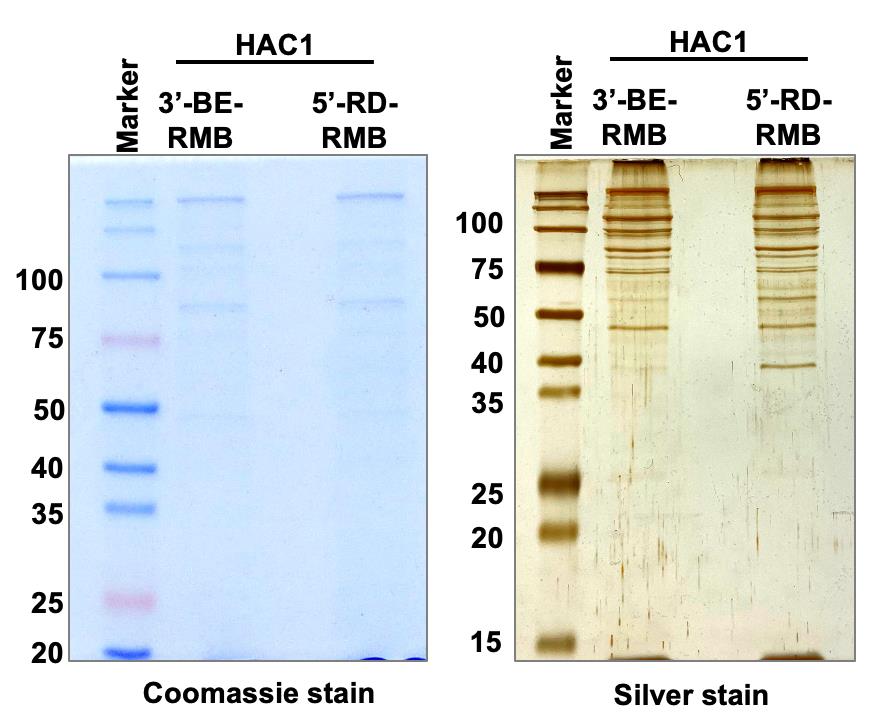
Figure 4. Pulldown of protein complex. Whole cell extract (WCE) from yeast cells expressing the biotinylated RNA (3’-BE-RMB or 5’-RD-RMB RNAs) was prepared and mixed with streptavidin agarose for 1 hour at 4°C. Proteins binding specifically to the 3’-BE- or 5’-RD-RMB mini-RNA were immuno-precipitated by streptavidin agarose and run on an SDS-polyacrylamide gel. The Coomassie blue- and Silver-stained gels are shown.
The cytosolic HAC1 mRNA in the budding yeast S. cerevisiae. contains an unusual intron. The intron is unusual because it is not spliced in the nucleus by the spliceosome, but is instead retained in the mRNA that is exported to the cytoplasm (Ruegsegger et al., 2001). This HAC1 intron interacts with its 5’-UTR to form an RNA duplex (RD), and this 5’-UTR-intron RD prevents translation initiation, thus keeping the mRNA in the translationally repressed form under normal conditions (Ruegsegger et al., 2001; Sathe et al., 2015). Under conditions of cellular stress, 3′-BE targets HAC1 mRNA to the endoplasmic reticulum (ER) stress site (Aragon et al., 2009), where the dual kinase RNase Ire1 cleaves the intron from the translationally repressed HAC1 mRNA (Cox et al., 1993; Mori et al., 1993; Gonzalez et al., 1999). However, the molecular mechanisms of 3′-BE-mediated mRNA transport and the RD-mediated translational repression are not yet defined, motivating us to identify both 3′-BE- and RD-protein complexes, and determine their functional role in targeting and translational de-repression of HAC1 mRNA.
Materials and Reagents
Materials
BasixTM 1.5 mL microcentrifuge tubes (Fisher Scientific, catalog number: 02-682-002)
FisherbrandTM 0.2 mL PCR Tubes (Fisherbrand, catalog number: 14-230-205)
50 mL centrifuge tubes (CellPro, catalog number: C5602)
50 mL centrifuge tubes (CellPro, catalog number: C5600)
250 mL Erlenmeyer glass flask (Corning, catalog number: 4980500)
150 mm × 15 mm Petri dish (Fisherbrand, catalog number: FB0875714)
4-Thiouracil (Alfa Aesar, catalog number: 591-28-6)
DL-Dithiothreitol (Sigma, catalog number: D9779)
0.5 mm zirconia beads (Fisher Scientific, catalog number: 11079105z)
Aprotinin (Sigma, catalog number: A1153)
Pepstatin A (Sigma, catalog number, P4265)
Protease inhibitor Tablet mini, EDTA-free (Thermo Fisher Scientific, catalog number: A32955)
Triton X-100 (Sigma, catalog number: T9284)
Phenylmethylsulfonyl fluoride (Sigma, catalog number: P7626)
Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad, catalog number: 5000006)
Streptavidin Sepharose® High Performance (GE Healthcare, catalog number: GE-17-5113-01)
SuperScript® III Reverse Transcriptase (Invitrogen, catalog number: 180-80-093)
RNaseOUT (Invitrogen, catalog number: P/W 100000840)
dNTPs (dATP, dGTP, dCTP, dTTP) (New England Biolabs Inc., catalog number: N0446S)
2× PCR master mixture (New England Biolabs Inc., catalog number: M0496S)
PierceTM Silver staining kit (Thermo Scientific, catalog number: 24612)
QIAzol reagent (QIAGEN, catalog number: 79306)
Chloroform (Sigma, catalog number: C2432)
Isopropanol (Sigma, catalog number: 19516)
RNase free water (Invitrogen, catalog number: 12183018)
Glycogen, RNA grade (Thermo Scientific, catalog number: R0551)
Ethanol (Pharmco Products, catalog number: 111000200)
Methanol (Fisher Chemical, catalog number: A412-4)
Acetic Acid, Glacial (Fisher Chemical, catalog number: A38-212)
Ethylenediaminetetraacetic acid (EDTA) (Sigma, catalog number: E9884)
Sodium acetate (Sigma, catalog number: S2889)
Bacteriological peptone (Thermo Scientific, catalog number: J20048-P5)
Yeast extract (Bio Basic, catalog number: 8013-01-2)
D-glucose (Sigma, catalog number: G8270)
Agar (Fisher Bioreagents, catalog number: BP9744-500)
Ultra-clear Quick dissolve Agarose (EZ Bioresearch, catalog number: S-1020-500)
Tris-Base (Fisher Bioreagents, catalog number: BP152-10)
Glycine (Sigma, catalog number: G8898)
Trichloroacetic acid (Sigma, catalog number: T6399)
Acetone (Sigma, catalog number: 179124)
Ammonium bicarbonate (AMBIC; Sigma, catalog number: A6141)
Iodoacetamide (IAA; Sigma, catalog number: I1149)
Sequencing Grade Modified Trypsin (Promega)
Lysyl-endopeptidaseR (Lys-C, FujiFilm Wako Pure Chemical Corporation)
Trifluoroacetic acid (TFA; Sigma, catalog number: T1647)
Formic acid (Sigma)
Sequencing Grade Modified Trypsin (Promega)
Sodium dodecyl sulfate (SDS) (Sigma, catalog number: 11667289001)
β-mercaptoethanol (β-ME) (Sigma, catalog number: 444203)
Diethyl pyrocarbonate (DEPC) (Sigma, catalog number: D5758)
Ethidium bromide (Sigma, catalog number: E1510)
Formaldehyde (Fisher Bioreagents: BP531-500)
Formamide (Sigma, catalog number: 47671)
DNase I (New England Biolabs Inc., catalog number: M0303S)
Yeast Nitrogen base (without amino acids) with ammonium sulphate (Sigma, catalog number: Y1251)
Amino acids (All amino acids from Sigma)
MOPS (Sigma, catalog number: M3183)
Potassium Chloride (Sigma, catalog number: P9541)
Magnesium acetate (Sigma, catalog number: M5661)
Brilliant Blue R (Sigma, catalog number: B7920)
Bromophenol blue (Sigma, catalog number: 114391)
Xylene Cyanol FF (Sigma, catalog number: X4126)
SC-uracil (complete synthetic medium without uracil)
YEPD (see Recipes)
Synthetic Complete (SC) medium with or without uracil or leucine (see Recipes)
1× PBS (see Recipes)
Buffer A (see Recipes)
Fixing solution for Coomassie staining (see Recipes)
Staining solution Coomassie staining (see Recipes)
Destaining solution Coomassie staining (see Recipes)
1× TE buffer (see Recipes)
10× MOPS buffer (see Recipes)
6× RNA loading dye (see Recipes)
Fixing solution for silver staining (see Recipes)
Sensitizer solution for silver staining (see Recipes)
Staining solution for silver staining (see Recipes)
Developer solution for silver staining (see Recipes)
D774, pRS426-ADH1-3’-BE-RMB (Ghosh et al., 2021)
D844, pRS425-ADH1-5’-RD-RMB (Ghosh et al., 2021)
X2159, MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ire1::NatMX hac1::KanMX, pRS426-ADH1-3’-BE-RMB: URA3
X2160, MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ire1::NatMX hac1::KanMX, pRS425-ADH1-5’-RD-RMB: LEU2
Equipment
Shaking incubator (Innova 42, New Brunswick Scientific)
Spectro linker- XL-1000 UV crosslinker (Spectronics Corporation)
Centrifuge (Eppendorf 5415R)
Centrifuge (Eppendorf 5810R)
UV transilluminator (UVITEC Cambridge)
Thermocycler-T100 (Bio-Rad)
NanoDrop-1000 Spectrophotometer (Thermo Scientific)
Incubator shaker (New Brunswick Innova 42)
Mini-100 Orbital shaker (Genie)
Diagger Vortex (Genie)
-80°C freezer (Sanyo)
Refrigerator (Sanyo)
Nutator shaker (Clay Adams)
High-Field Hybrid Ion Trap-Orbitrap Mass Spectrometer (Thermo Scientific Orbitrap Elite)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Uppala, J. K., Ghosh, C., Sabat, G. and Dey, M. (2022). Pull-down of Biotinylated RNA and Associated Proteins. Bio-protocol 12(4): e4331. DOI: 10.21769/BioProtoc.4331.
- Ghosh, C., Uppala, J. K., Sathe, L., Hammond, C. I., Anshu, A., Pokkuluri, P. R., Turk, B. E. and Dey, M. (2021). Phosphorylation of Pal2 by the protein kinases Kin1 and Kin2 modulates HAC1 mRNA splicing in the unfolded protein response in yeast. Sci Signal 14(684).
分类
分子生物学 > RNA > RNA-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













