- EN - English
- CN - 中文
NBD-lipid Uptake Assay for Mammalian Cell Lines
哺乳动物细胞系的 NBD 脂质摄取测定
发布: 2022年02月20日第12卷第4期 DOI: 10.21769/BioProtoc.4330 浏览次数: 4696
评审: Ralph Thomas BoettcherGuillaume LenoirAnonymous reviewer(s)

相关实验方案
外周血中细胞外囊泡的分离与分析方法:红细胞、内皮细胞及血小板来源的细胞外囊泡
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
2025年11月05日 1435 阅读
Abstract
All eukaryotic cells are equipped with transmembrane lipid transporters, which are key players in membrane lipid asymmetry, vesicular trafficking, and membrane fusion. The link between mutations in these transporters and disease in humans highlights their essential role in cell homeostasis. Yet, many key features of their activities, their substrate specificity, and their regulation remain to be elucidated. Here, we describe an optimized quantitative flow cytometry-based lipid uptake assay utilizing nitrobenzoxadiazolyl (NBD) fluorescent lipids to study lipid internalization in mammalian cell lines, which allows characterizing lipid transporter activities at the plasma membrane. This approach allows for a rapid analysis of large cell populations, thereby greatly reducing sampling variability. The protocol can be applied to study a wide range of mammalian cell lines, to test the impact of gene knockouts on lipid internalization at the plasma membrane, and to uncover the dynamics of lipid transport at the plasma membrane.
Graphic abstract:

Internalization of NBD-labeled lipids from the plasma membrane of CHO-K1 cells.
Background
A remarkable feature of many biological membranes is that their phospholipids are asymmetrically distributed across the lipid bilayer, a phenomenon known as transbilayer lipid asymmetry. This lipid asymmetry is essential for several vital cellular functions, including regulation of membrane protein activity, signaling, and vesicle formation in the secretory and endocytic pathways. Thus, establishing and regulating lipid asymmetry is crucial for cells, and a number of membrane proteins have evolved to fulfill the function of cross-bilayer lipid transporters. These transporters include ATP-dependent flippases and floppases—which catalyze the inward movement of lipids from the extracellular/luminal leaflet to the cytoplasmic leaflet, and the outward movement of lipids from the intracellular leaflet to the extracellular/ luminal leaflet, respectively, and ATP-independent scramblases (Holthuis and Levine, 2005; Contreras et al., 2010). Despite their fundamental cellular importance, key aspects of how these lipid transporters operate await elucidation.
A subgroup of P-type ATPases, the P4-ATPases, emerged as a major group of lipid flippases that form heterodimeric complexes with members of the Cdc50 (cell division control 50) protein family (Lopez-Marques et al., 2014). Mutations in these transporters generate impairments in physiological processes and, in humans they have been linked to diseases such as intrahepatic cholestasis and cerebellar ataxia (van der Mark et al., 2013). While initially characterized as aminophospholipid flippases, recent studies of individual family members from yeast, parasites such as Leishmania, plants, and mammalian cells show that P4-ATPases differ in their substrate specificities and mediate transport of a broader range of lipid substrates, including lysophospholipids, synthetic alkylphospholipids, and glycolipids (Roland et al., 2019; Shin and Takatsu, 2019).
Characterization of phospholipid movement at a quantitative level in the plasma membrane of eukaryotic cells is frequently based on fluorescent lipids with a covalently linked 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD) group in the sn-2 position. These lipid analogs have a NBD group attached to a short fatty acid chain (C6) and maintain most of the properties of endogenous phospholipids, except that they are more water-soluble, which facilitates incorporation from the medium into the outer monolayer of the plasma membrane. Transport of these probes is usually monitored by extracting the residual fraction of analogs not transported across the membrane with bovine serum albumin (BSA). As BSA extracts all analogs from the exoplasmic monolayer of the plasma membrane, the inaccessible fraction reflects analogs that have been redistributed across the plasma membrane into cells.
The protocol presented here utilizes flow cytometry to study NBD-lipid internalization at a quantitative level in mammalian cells, exemplified on Chinese hamster ovary-K1 (CHO-K1) cells, and has been applied by us to fibroblasts (Pomorski et al., 1996), lymphocytes (Fischer et al., 2006), and myoblasts (Grifell-Junyent et al., 2022). For lipid uptake assays optimized for fungi and plants, the reader is referred to previously published protocols (Jensen et al., 2016; López-Marqués and Pomorski, 2021). The protocol includes the preparation of NBD-lipids, labelling of cells with NBD-lipids, flow cytometry measurements, and data analysis. Additionally, cells are subjected to lipid analysis via thin-layer chromatography (TLC) to assess metabolic conversion of the NBD-lipids. This protocol can be easily adapted to parasites such as Toxoplasma and Leishmania (Weingartner et al., 2011; dos Santos et al., 2013; Chen et al., 2021), and has a broad range of applications, including: i) screening of mammalian cell lines for their lipid uptake profile, ii) testing the impact of gene knockouts on lipid internalization at the plasma membrane, and iii) uncovering the dynamics of lipid transport at the plasma membrane.
Things to consider before starting
Choice of NBD-lipid
The NBD-lipids need to fulfill three important requirements: (i) they cannot be modified at their polar head group, as this is the key structural element for substrate recognition by ATP-dependent flippases and floppases (Theorin et al., 2019); (ii) they have to incorporate readily into the outer plasma membrane, to get the time zero for the uptake kinetic; and (iii) they have to be extractable by BSA. These requirements are best met by lipid analogs in which one fatty acid has been replaced by a short chain of six carbons (C6) carrying the fluorescent NBD moiety in the sn-2 position of the glycerophospholipids, or linked by an amide bond to the ceramide backbone, as well as lyso-glycerophospholipid derivates labeled at the sn-1 position (Figure 1).
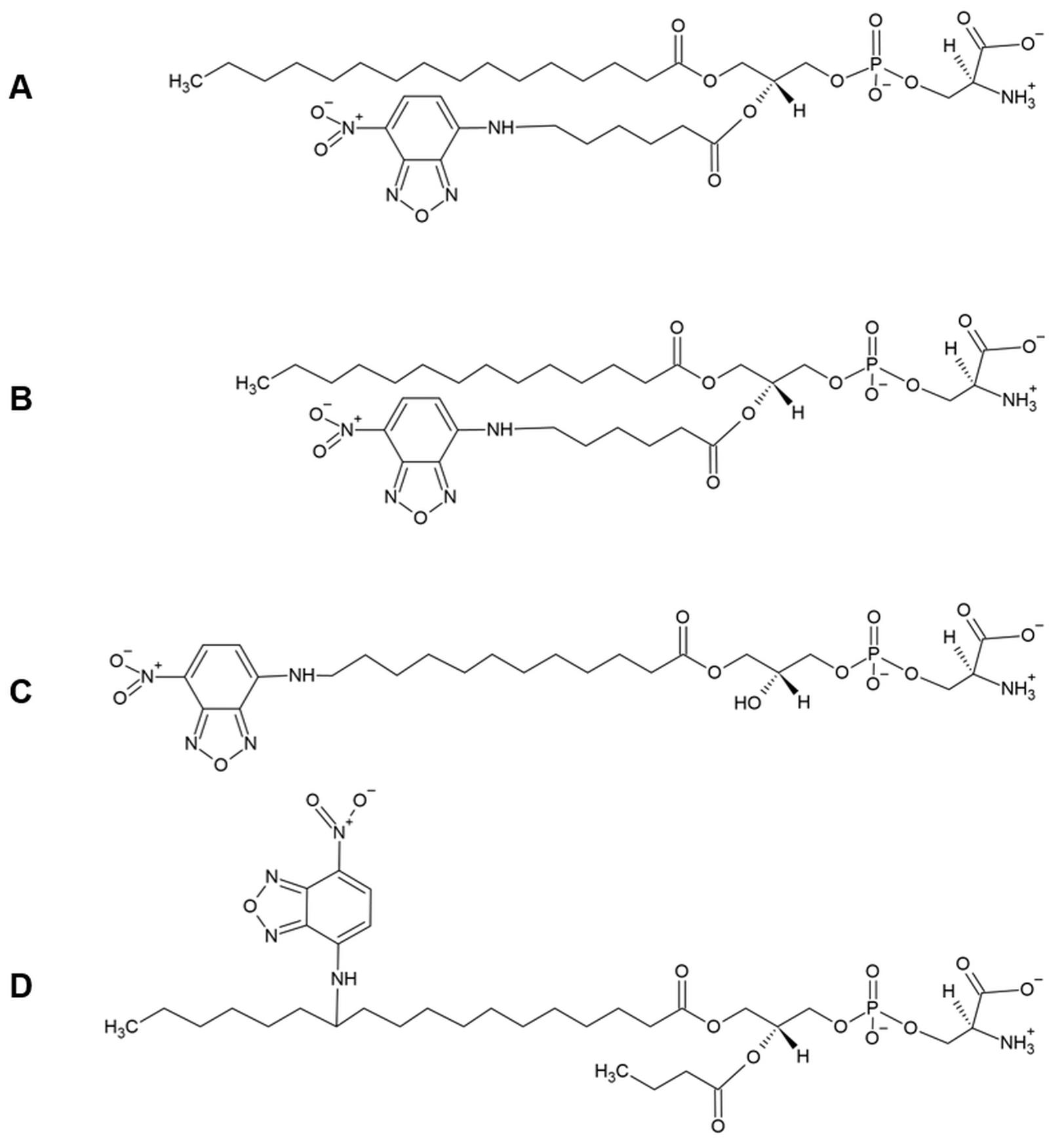
Figure 1. Chemical structure of NBD-lipids used in lipid uptake assays. Fluorescent analogs of phosphatidylserine with different acyl chain lengths and positions of the NBD moiety. A) 1-palmitoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphoserine (C16:0-C6:0 NBD-PS). In these analogs, one fatty acid has been replaced by a short chain of six carbons (C6) carrying the fluorescent NBD moiety in the sn-2 position, whereas the sn-1 chain is composed of sixteen carbons (C16). B) 1-myristoyl-2-{6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphoserine (C14:0-C6:0 NBD-PS); similar to the analog in (A), but the sn-1 chain is composed of fourteen carbons (C14). C) 1-NBD-dodecanoyl-2-hydroxy-sn-glycero-3-phospho-serine (NBD-lyso-PS). In these analogs, the C12 sn-1 acyl chain carries the NBD moiety. D) The NBD moiety is attached to the carbon twelve of a C18 sn-1 acyl chain, whereas the sn-2 chain is composed of four carbons (Colleau et al., 1991).Metabolic conversion of the NBD-lipids
In nucleated cells, lipid internalization is not only affected by transbilayer movement and intracellular membrane trafficking, but also by lipid metabolism. Some lipid analogs are known to be actively metabolized by phospholipase activities at the cell surface. Examples are the hydrolysis of NBD- phosphatidic acid to NBD-diacylglycerol (Pagano and Longmuir, 1985), and the hydrolysis of NBD-sphingomyelin to NBD-ceramide, both occurring at the plasma membrane. The fluorescent analogs of diacylglycerol and ceramide undergo rapid spontaneous transbilayer movement, and therefore label intracellular membranes (Pagano and Sleight, 1985).
Thus, the conversion of an NBD-lipid may be followed by rapid spontaneous movement of its metabolic products and should always be critically evaluated. Another frequent modification is the hydrolysis of the NBD-lipid analogs into lyso-derivates by phospholipase A2 activities, which results in the removal of the fatty acid attached to the sn-2 position. The labeled C6 fatty acids liberated are released into the medium, hampering quantitative analysis of NBD-lipid internalization. To block this conversion, the assay is typically performed in the presence of phospholipase inhibitors (Pomorski et al., 1996; Fischer et al., 2006; Grifell-Junyent et al., 2022).
Therefore, it is highly recommended to analyze the degree of NBD-lipid conversion using, for example, lipid extraction in organic solvents followed by thin layer chromatography (see under Procedure, section D).Lipid internalization by endocytosis
Lipid analogs inserted into the outer plasma membrane leaflet can also be internalized by endocytosis. To suppress uptake by endocytosis, the assay is typically performed at 20°C or below.
Materials and Reagents
Mammalian cell culture
In this study, we used Chinese hamster ovary-K1 cells (CHO-K1; Cell number: RCB0285, Riken BRC, Japan) kindly provided by Dr. Kentaro Hanada, that were cultured in cell culture medium (see Recipes).
Sterile serological pipettes (e.g., Serological pipettes of 5 mL, 10 mL and 25 mL; Sarstedt, catalog numbers: 86.1253.001, 86.1254.001, 86.1685.001)
Sterile culture vessels T-75 flasks (e.g., Sarstedt, catalog number: 83.3911)
Basal cell culture medium (e.g., high glucose DMEM; Sigma-Aldrich, catalog number: D6546)
Fetal bovine serum (FBS; e.g., Sigma-Aldrich, catalog number: F4135)
L-glutamine stock of 200 mM (e.g., Sigma-Aldrich, catalog number: G7513)
Penicillin-streptomycin (e.g., Sigma-Aldrich, catalog number: P4333)
Trypsin-EDTA solution (e.g., Sigma-Aldrich, catalog number: T3924)
Hanks’ Balanced Salt solution (HBSS; e.g., Sigma-Aldrich, catalog number: H6648)
Trypan Blue Solution, 0.4% (Thermo Fisher Scientific, catalog number: 15250061)
Tyrode's balanced salt solution (TBSS; see Recipes)
Preparation of NBD-lipids
C16:0-C6:0 NBD-lipids purchased in chloroform including:
NBD-PC (Avanti Polar Lipids, catalog number: 810130)
NBD-PE (Avanti Polar Lipids, catalog number: 810153)
NBD-PS (Avanti Polar Lipids, catalog number: 810192)
NBD-SM (Avanti Polar Lipids, catalog number: 810218)
Dimethyl sulfoxide (DMSO; Carl Roth, catalog number: 4720.4)
Labelling of cells with NBD-lipids
3-(-4-octadecyl)-benzoylacrylic acid (OBAA; Sigma-Aldrich, catalog number: SML0075)
Phenylmethylsulfonyl fluoride (PMSF; Sigma-Aldrich, catalog number: p7626)
Note: OBAA is a potent inhibitor of phospholipase A2 (Eintracht et al., 1998); PMSF has been reported to inhibit phospholipases, as well as some esterases (James, 1978; Estevez et al., 2012; Gadella and Harrison, 2000).
Analysis of NBD-lipid uptake and metabolism
PYREX® 11 mL screw cap culture tubes, 16 × 100 mm (Corning, catalog number: 9825-16), and centrifuge glasses DURAN® with conical bottoms, 12 mL (Carl Roth, catalog number: K211.1). If not using a cap (see point D2), centrifuge glasses DURAN® with round bottoms, 12 mL (Carl Roth, catalog number: C102.1) can also be used instead.
Tube caps with polypropylene for centrifuge glasses (Ohemen Labor, catalog number: 6702588). Using of caps is optional.
Polypropylene tubes of 15 mL and 50 mL capacity (e.g., Falcon tubes, Sarstedt, catalog numbers: 62.554.502 and 62.547.254)
1.5-mL microcentrifuge tubes (Sarstedt, catalog number: 72.690.001)
Tubes for flow cytometer (e.g., Sarstedt, catalog number: 55.484)
Reusable glass media bottles with cap, 250 mL and 1 L (e.g., Thermo Fisher Scientific, catalog numbers: 15456113 and 15486113)
Graduated pipette serological, 5 mL (Sigma-Aldrich, catalog number: BR27112)
MacromanTM pipette (Gilson, catalog number: F110120)
Disposable glass Pasteur pipettes, 230 mm (VWR, catalog number: 612-1702)
Glass Pasteur pipette bulb (VWR, catalog number: 470123-222)
Bovine serum albumin, essentially fatty acid free (BSA; Sigma-Aldrich, catalog number: A6003)
Note: Fatty acid-free BSA allows for efficient extraction of NBD-lipids from cellular membranes, due to the unoccupied fatty acid binding sites.
Propidium iodide ≥94.0% (HPLC) (PI; Sigma-Aldrich, catalog number: P4170)
Flow cytometer cleaning solution (e.g., Sysmex, catalog number: 04-4009_R)
Flow cytometer sheath fluid (e.g., Sysmex, catalog number: 04-4007_R)
Chloroform 99-99.4% ethanol-stabilized and certified for absence of phosgene and HCl (VWR, catalog number: 22711.290)
Ethanol absolute ≥99.8% (VWR, catalog number: 20821.321)
Methanol ≥99.8% (VWR, catalog number: 20847)
Triethylamine (Carl Roth, catalog number: X875.3)
Thin layer chromatography (TLC) Silica gel 60, 10 × 20 cm (Merck, Darmstadt, Germany, catalog number: 1.05626.0001)
Media and buffers
Cell culture medium (see Recipes)
TBSS buffer (see Recipes)
NBD-lipid stocks (see Recipes)
PMSF stock of 200 mM (see Recipes)
OBAA stock of 5 mM (see Recipes)
BSA (20% w/v) in TBSS (see Recipes)
PI stock of 1 mg mL-1 (see Recipes)
Alkaline running buffer (see Recipes)
Equipment
Analytical balance (e.g., Sartorius Entris-i II, 220 g/0.1 mg, Buch Holm, catalog number: 4669128)
Eppendorf Research® plus pipettes P20, P200, P1000 (Eppendorf, catalog numbers: 3123000039, 3123000055, 3123000063)
Pipette tips 10 µL, 200 µL, 1,000 µL (Sarstedt, catalog numbers: 70.760.002, 70.3030.020, 70.3050.020)
Neubauer counting chamber (improved Dark lines, 0.1 mm) and cover glasses (20 mm × 26 mm × 0.4 mm)
Flow cabinet to work with organic solvents
Biological safety cabinet certified for handling of biological materials (e.g., Herasafe KSP Class II Biological Safety Cabinets, Thermo Fisher Scientific)
Hamilton 700 Series Syringes 25 µL, 100 µL, 1,000 µL (Hamilton Company, Nevada, USA)
Centrifuge with rotor for 15 mL and 50 mL polypropylene tubes (e.g., Eppendorf 5810 R; Wesseling, Germany)
Autoclave sterilizer (e.g., Systec VX-65, Systec, Linden, Germany)
Water distillation system
Incubator with humidity and gas control to maintain 37°C and 95% humidity in an atmosphere of 5% CO2 in air (e.g., Binder, Tuttlingen, Germany)
Inverted light microscope equipped with a 10× objective (HI PLAN I 10×/0.22 PH1; Leica DMi1, Mannheim, Germany)
Water bath (e.g., WPE45 Memmert, Schwabach, Germany) for mammalian cells and for NBD-lipid labelling (Julabo CORIO C-BT5, catalog number: 9011305)
Vortex mixer (e.g., Vortex Genie 2 Scientific Industries Inc., catalog number: SI-0236)
Vacuum Pump V-100 with Interface I-100 (Buchi, catalog numbers: 11593636 and 11593655D)
Glass desiccator Boro 3.3 with socket in lid, 20 cm, including stopcock (BRAND GmbH, catalog number: 65238)
Tubing (BRAND GmbH, catalog number: 143275)
Developing chamber for TLC (Roth Carl, catalog number 3133.1)
Freezers at -20°C and -80°C
Refrigerator
Fluorescence Imager
For this protocol, a Chemidoc MP Imaging System (Bio-Rad, Munich, Germany) with a 530/28 filter and light Blue Epi illumination was used.
Flow cytometer
For this protocol, a CyFlow® SL flow cytometer (Sysmex Partec, Münster, Germany) was used, equipped with a solid-state laser of 488 nm.
Computer with monitor (e.g., DELL U2415)
Software
FloMax® software (Sysmex Partec)
FlowJoTM v10.0.7 Software (FlowJo)
Image LabTM software (Bio-Rad)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Herrera, S. A., Grifell-Junyent, M. and Pomorski, T. G. (2022). NBD-lipid Uptake Assay for Mammalian Cell Lines. Bio-protocol 12(4): e4330. DOI: 10.21769/BioProtoc.4330.
分类
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
发育生物学 > 细胞生长和命运决定 > 分化
生物物理学 > 显微技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link