- EN - English
- CN - 中文
Restimulation-Induced Cell Death (RICD): Methods for Modeling, Investigating, and Quantifying RICD Sensitivity in Primary Human T Cells via Flow Cytometric Analysis
再刺激诱导的细胞死亡 (RICD):通过流式细胞术分析在原代人类 T 细胞中建模、调查和量化 RICD 敏感性的方法
发布: 2022年02月20日第12卷第4期 DOI: 10.21769/BioProtoc.4326 浏览次数: 4514
评审: Giusy TornilloAnonymous reviewer(s)

相关实验方案

研究免疫调控血管功能的新实验方法:小鼠主动脉与T淋巴细胞或巨噬细胞的共培养
Taylor C. Kress [...] Eric J. Belin de Chantemèle
2025年09月05日 3445 阅读
Abstract
When the body mounts an immune response against a foreign pathogen, the adaptive arm of the immune system relies upon clonal expansion of antigen-specific T cell populations to exercise acquired effector and cytotoxic functions to clear it. However, T cell expansion must be modulated to effectively combat the perceived threat without inducing excessive collateral damage to host tissues. Restimulation-induced cell death (RICD) is an apoptotic program triggered in activated T cells when an abundance of antigen and IL-2 are present, imposing a negative feedback mechanism that constrains the growing T cell population. This autoregulatory process can be detected via increases in caspase activation, Annexin V binding, and loss of mitochondrial membrane potential. However, simple changes in T cell viability through flow cytometric analysis can reliably measure RICD sensitivity in response to T-cell receptor (TCR) restimulation. This protocol describes the in vitro polyclonal activation, expansion, and restimulation of human primary T cells isolated from donor peripheral blood mononuclear cells (PBMC). This simple procedure allows for accurate quantification of RICD via flow cytometry. We also describe strategies for interrogating the role of specific proteins and pathways that may alter RICD sensitivity. This straightforward protocol provides a quick and dependable tool to track RICD sensitivity in culture over time while probing critical factors that control the magnitude and longevity of an adaptive immune response.
Graphic abstract:
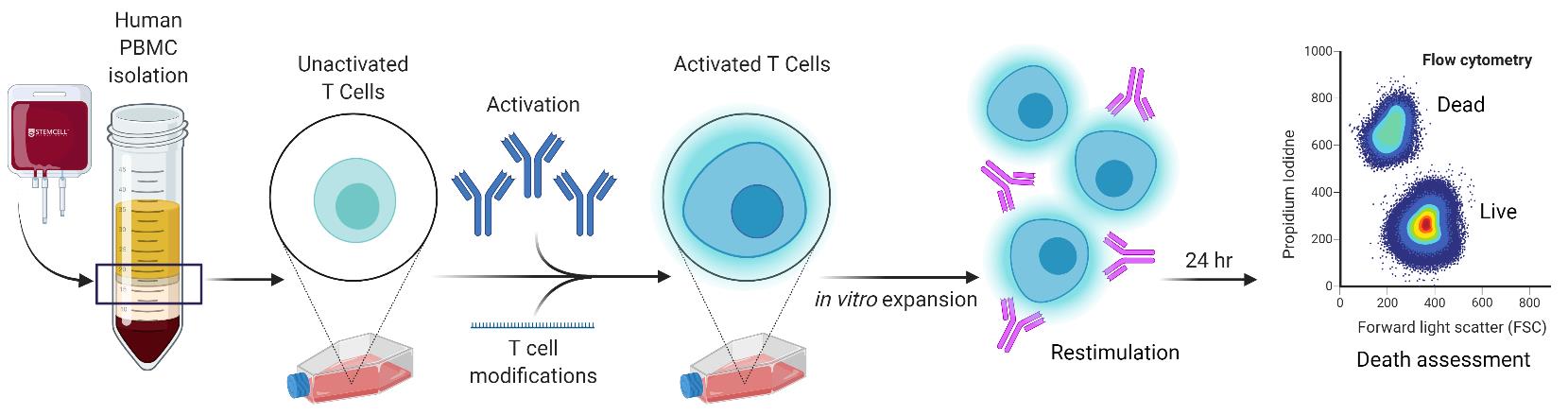
In-vitro simulation of restimulation-induced cell death in activated human T cells.
Background
Adaptive immunity requires the selective engagement of highly specific T cell clones to respond to “foreign invaders”, including microbial pathogens and tumor cells. An effective T cell response demands a fine balance of T cell receptor (TCR) and costimulatory/coinhibitory signals in order to regulate both expansion and contraction. The ability to raise powerful “armies” of CD4 and/or CD8 effector T cells from naïve or memory compartments ultimately determines whether a foreign invader will be neutralized or eliminated. Concurrently, the immune system must regulate these deadly forces to guard against deleterious immunopathologies associated with lymphoproliferation, hyperinflammation, and autoimmunity. Several checkpoints and signaling molecules help determine the size and duration of a T cell effector response after activation, including propriocidal apoptotic programs. One such self-regulatory pathway used to constrain expanding T cell populations is restimulation-induced cell death (RICD) (Nagy et al., 2010; Snow et al., 2010). RICD relies on the presence of antigen and interleukin-2 (IL-2) to induce programmed cell death in a subset of activated T cells following TCR re-engagement (Figure 1). This is distinct from cytokine withdrawal-induced death, an intrinsic apoptotic program triggered by IL-2 deprivation after pathogen clearance, and responsible for most effector cell contraction (Snow et al., 2010). Understanding the mechanisms that govern RICD sensitivity can help to elucidate if and how abnormal T cell dynamics might contribute to lymphoproliferative disorders, lymphomas, autoimmune diseases, infections, and other states of immune dysregulation.
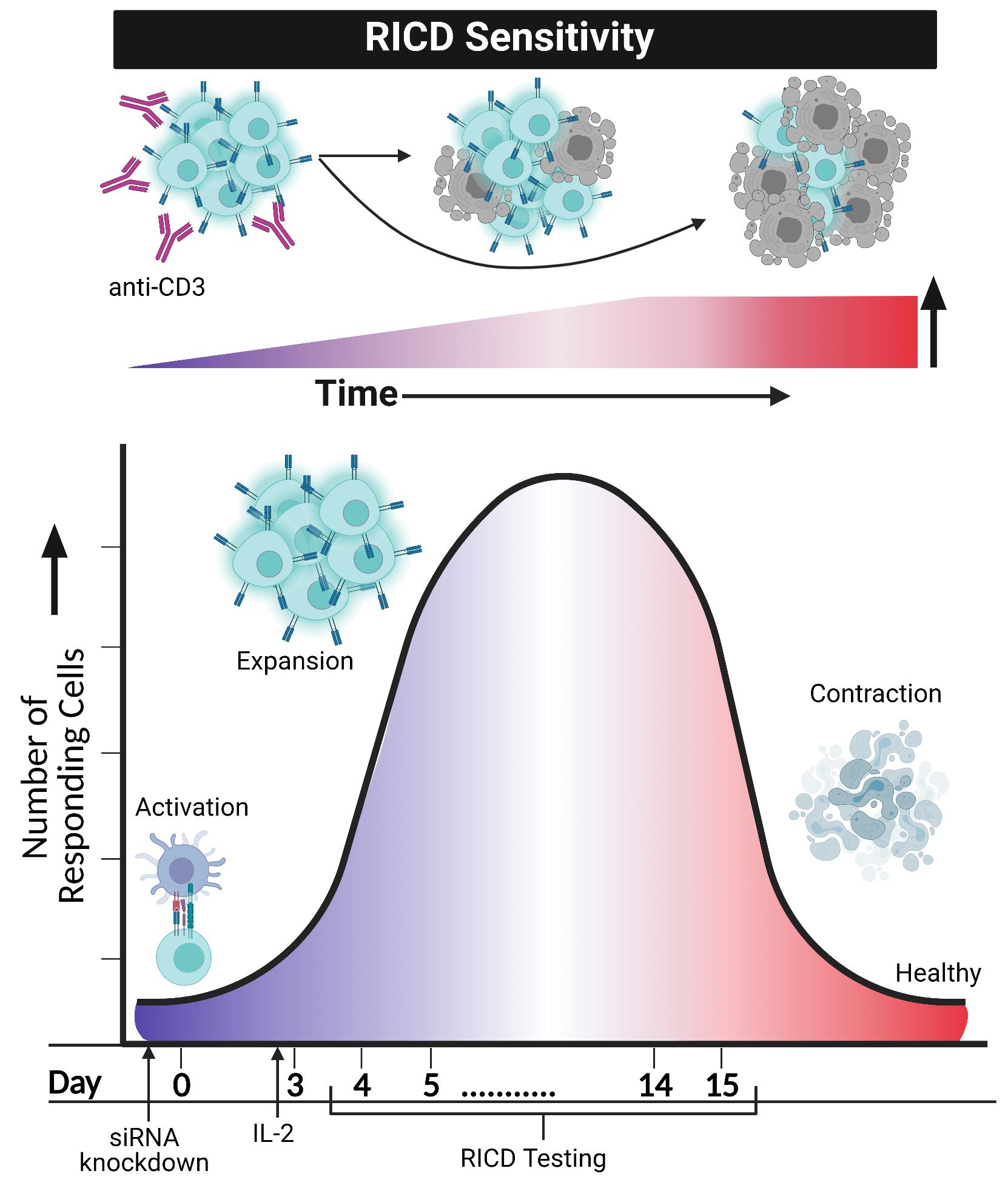
Figure 1. RICD sensitivity in activated T cells increases over time and may be imitated and investigated in an in vitro format. Restimulating activated T cells with anti-CD3 in the presence of IL-2 will cause a certain percentage of T cells to induce apoptosis. Anti-CD28 does not seem to be a contributor to this phenomenon, despite its requirement for T cell activation. Simulation of RICD through in vitro assays may be performed, testing cells anywhere from day 4 post-activation to day 15. Modulation of T cells, such as siRNA knockdown of various proteins, can occur before day 0 activation with CD3/CD28. After IL-2 addition to in vitro cultures of T cells on day 3, RICD may be tested in “early stage” effector cells (day 4) or “late stage” effector cells (day 14). Comparing control and siRNA knockdown cells helps elucidate mechanistic and molecular requirements for RICD sensitivity. Figure created using Biorender.com.
Apoptosis is utilized through T cell development and differentiation to dispose of nonfunctional, autoreactive, or excess cells (Zhang et al., 2005). Therefore, delineating mechanisms of T cell homeostasis and RICD is critical for understating T cell-driven immunopathology. In humans, autoimmune lymphoproliferative syndrome (ALPS) and X-linked lymphoproliferative disease 1 (XLP-1) are associated with apoptosis defects linked to loss-of-function or null mutations in Fas/FasL and SAP (SLAM-associated protein), respectively (Snow et al., 2009; Nagy et al., 2010; Nourse et al., 2010). ALPS patients exhibit enlarged lymph nodes and spleens, with a predilection for autoimmunity and lymphoma development. XLP-1 patients experience an overreactive and life-threatening accumulation of CD8 effector T cells in response to infection by Epstein Barr virus (EBV), which we specifically linked to RICD resistance in the absence of SAP (Snow et al., 2009). Aside genetic pre-dispositions, the tumor microenvironment also forces antigen-specific TILs to more readily succumb to apoptosis (including RICD), limiting tumor control and highlighting the significance of this pathway in multiple environments.
Early descriptions of RICD were termed “activation-induced cell death (AICD)”, initially observed as a decrease in growth of murine T cell hybridomas after antigen stimulation in a dose-dependent manner (Ashwell et al., 1987a and 1987b). However, further studies introduced “RICD” as a more relevant descriptor for this phenomenon, which only occurs in previously activated and proliferating effector T cells that experience TCR re-ligation in the presence of IL-2 (Snow et al., 2010). Early investigations indicated that RICD was specifically restricted to cycling T cells and attributed to TCR-induced FASL induction, which triggered autocrine/paracrine FAS-dependent apoptosis particularly in CD4 T cells (Boehme and Lenardo, 1993; Brunner et al., 1995; Dhein et al., 1995; Lenardo et al., 1999). Additional pro-apoptotic molecules, such as BIM and perforin/granzymes, were later implicated in RICD of CD8 T cells (Mateo et al., 2007; Snow et al., 2008). Our subsequent work defined how SAP (SH2D1A) potentiates TCR signal strength by displacing SHP-1 (SH2 domain-containing protein tyrosine phosphatase) and recruiting more LCK (lymphocyte-specific protein tyrosine kinase) to the SLAM family receptor NK, B, and T cell antigen (NTB-A), as well as inhibiting diacylglycerol kinase alpha (DGKa), to promote the expression of several pro-apoptotic molecules (FASL, BIM, NOR1, NUR77) involved in executing RICD (Katz et al., 2014; Ruffo et al., 2016). Moreover, natural suppression of SAP and FASL expression in regulatory T cells renders them resistant to RICD, enforced by the master regulator FOXP3 (Katz et al., 2018). More recently, we discovered that cellular metabolic processes, including glycolysis and fatty acid synthesis, can influence the apoptotic susceptibility of effector T cells (Larsen et al., 2017; Voss et al., 2017 and 2019). Despite these mechanistic insights into RICD of terminally-differentiated (i.e., “late-stage”) effector T cells, it remains unclear how early activated T cells undergoing clonal expansion (i.e., “early stage”) are spared from premature RICD in the presence of abundant antigen and IL-2. We recently described how transient FOXP3 expression can protect newly-activated human conventional T cells from RICD by promoting basal autophagy and suppressing p53 (Voss et al., 2021). The protocol described herein is based on our recent paper describing how TIM-3, a co-signaling protein often linked to CD8 T cell exhaustion, can influence subtle and temporal changes in RICD sensitivity (Lake et al., 2021).
Our original in vitro RICD protocol for human late-stage effector T cells was described previously (Katz and Snow, 2013). Here, we expand on a modified protocol to measure RICD sensitivity at different stages of the T cell response, including the interrogation of specific target proteins like TIM-3 to elucidate their role in tuning RICD sensitivity. Our method offers a quick and easy way to model human T cell activation and RICD induction in an in vitro setting, which may be coupled with other apoptotic readouts such as Annexin V staining, caspase activation, and mitochondrial depolarization (Wlodkowic et al., 2011). Clinicians and research scientists may utilize this procedure to evaluate and manipulate RICD sensitivity over time in healthy donor T cells, or test for abnormal RICD in patients with lymphoproliferative disease, or other primary immune regulation disorders (PIRDs). Through this approach, our method has proven highly valuable in delineating myriad molecular mechanisms that govern T cell homeostasis via RICD.
Materials and Reagents
50 mL conical tubes (sterile) (Genesee, catalog number: 28-106)
15 mL conical tubes (sterile) (Genesee, catalog number: 28-103)
9” Glass Pasteur pipettes (Daigger, catalog number: EF20402C)
10 mL serological pipettes (Genesee, catalog number: 12-104)
5 mL serological pipettes (Genesee, catalog number: 12-102)
6 well tissue culture plates (Diagger, catalog number: EF24881)
Vented tissue culture flasks (Genesee, catalog number: 25-209)
96 well round bottom plates (Genesee, catalog number: 25-109)
1.1 mL polypropylene tubes (USA Scientific, catalog number: 1412-1000)
5 mL round bottom tubes for flow cytometry (VWR, catalog number: 60819-728)
16 mL sterile polystyrene culture tubes (Genesee, catalog number: 21-129)
Mouse IgG1 Isotype Control SAFIRE (Bio-gems, 44212-25)
Whole blood or “buffy coat” (i.e., PBMC enriched fraction of whole blood post centrifugation)
Ficoll-Paque PLUS (GE Healthcare, catalog number: 17-1440-03)
1× Phosphate-buffered saline (PBS) (VWR, catalog number: 95042-486)
ACK Lysis Buffer (VWR, catalog number: 10128-806)
RPMI-1640 w/ Glutamine (VWR, catalog number: 95042-508)
Fetal Bovine Serum (Milipore Sigma, catalog number: F0926-500)
Penicillin-Streptomycin (Thermo Fisher, catalog number: 15140163)
Trypan blue 4 g/L (VWR, catalog number: K940-100ML)
Immunocult CD3/CD28/CD2 T Cell Activator (Stem Cell Technologies, catalog number: 10970)
Anti-human CD3 antibody, clone HIT3a NA/LE (BD Biosciences, catalog number: 555336)
Anti-human CD28 antibody (BD Biosciences, catalog number: 555725)
Anti-CD3 antibody OKT3 (Bio-gems, catalog number: 01521-05)
Recombinant human IL-2 (Peprotech, catalog number: 200-02)
Propidium iodide (Thermo Fisher, catalog number: P1304MP)
Amaxa P3 Primary Cell 4D-Nucelofactor X Kit L (Lonza, V4XP-3024) (included: Lonza P3 buffer, Lonza pipettes, and Lonza cuvettes)
RoboSepTM Buffer (Stem Cell Technologies, catalog number: 20104)
Human CD8+ T cell Isolation Kit (Stem Cell Technologies, catalog number: 17953)
TIM-3 siRNA (Thermo Fisher, catalog number: 4392420) (stored at -20°C, aliquoted)
Nonspecific siRNAcontrol (Thermo Fisher, catalog number: 12935300) (stored at -20°C, aliquoted)
TIM-3 blocking monoclonal antibody, clone F38-2E2 (Biolegend, catalog number: 35004)
Recombinant human Tim-3-FC Chimera (R&D Systems, catalog number: 2365-TM-050)
200 Proof Ethanol (Decon Labs Inc., catalog number: 2716)
Ultra-pure water (university provided)
70% ethanol (see Recipes)
Complete RPMI Media (see Recipes)
Antibiotic-free RPMI Media (see Recipes)
Recombinant human IL-2 solution (rIL-2) (see Recipes)
siRNA aliquots (see Recipes)
PI solution (see Recipes)
Equipment
Centrifuge Sorvall Legend X1XTR (Thermo Fisher, catalog number: 75004539)
Class II A2 biosafety cabinet (for sterile workflow)
Accuri C6 Flow Cytometer (Becton Dickenson)
Vortex-Genie 2 (Scientific Industries Inc., catalog number: SI-0236)
4D NucleofectorTM Core Unit and 4D NucleofectorTM X Unit (Lonza, catalog number: AAF-1002B and AAF-1002X) (included: Lonza cuvette holder)
“The Big Easy” EasySepTM Magnet (Stem Cell Technologies, catalog number: 18001)
Pipette Aid (Genesee, catalog number: 33-901)
Shel Lab 37°C water bath (Sheldon Manufacturing Inc., catalog number: SWB15)
HeracellTM 150i 5% CO2 Incubator (Thermo Fisher, catalog number: 50116048)
Cell counter or hemocytometer
Software
Accuri C6 Flow Cytometer Software (Becton Dickenson)
FlowJo (Becton Dickenson, https://www.flowjo.com/)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Pohida, K., Lake, C. M., Yee, D. and Snow, A. L. (2022). Restimulation-Induced Cell Death (RICD): Methods for Modeling, Investigating, and Quantifying RICD Sensitivity in Primary Human T Cells via Flow Cytometric Analysis. Bio-protocol 12(4): e4326. DOI: 10.21769/BioProtoc.4326.
分类
免疫学 > 免疫细胞功能 > 淋巴细胞
免疫学 > 免疫细胞分离 > 白细胞
细胞生物学 > 细胞活力 > 细胞死亡
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link