- EN - English
- CN - 中文
Studying Chemotactic Migration in Dunn Chamber: An Example Applied to Adherent Cancer Cells
在Dunn Chamber中研究趋化迁移:应用于粘附癌细胞的案例
发布: 2022年02月05日第12卷第3期 DOI: 10.21769/BioProtoc.4316 浏览次数: 2937
评审: Alak MannaBeom K ChoiAnonymous reviewer(s)
Abstract
Cell migration is a vital process in the development of multicellular organisms. When deregulated, it is involved in many diseases such as inflammation and cancer metastisation. Some cancer cells could be stimulated using chemoattractant molecules, such as growth factor Heregulin β1. They respond to the attractant or repellent gradients through a process known as chemotaxis. Indeed, chemotactic cell motility is crucial in tumour cell dissemination and invasion of distant organs. Due to the complexity of this phenomenon, the majority of available in vitro methods to study the chemotactic motility process have limitations and are mainly based on endpoint assays, such as the Boyden chamber assay. Nevertheless, in vitro time-lapse microscopy represents an interesting opportunity to study cell motility in a chemoattracting gradient, since it generates large volume image-based information, allowing the analysis of cancer cell behaviours. Here, we describe a detailed time-lapse imaging protocol, designed for tracking T47D human breast cancer cell line motility, toward a gradient of Heregulin β1 in a Dunn chemotaxis chamber assay. The protocol described here is readily adapted to study the motility of any adherent cell line, under various conditions of chemoattractant gradients and of pharmacological drug treatments. Moreover, this protocol could be suitable to study changes in cell morphology, and in cell polarity.
Background
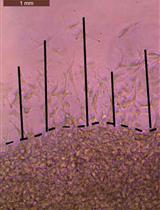
Directed migration is a key process implicated in physiological and in pathological phenomena, like wound-healing and metastasis. When a cell polarizes and migrates in a directed way, in response to a ligand simulation, it is called chemotactic migration (Stuelten et al., 2018). In adherent and non-adherent cells, migration engages cytoskeletal components and regulators in different manners; chemotactic migration involves close but dissimilar mechanisms, depending on cell types and experimental conditions (Entschladen et al., 2005). To analyse chemotactic migration, various devices have been generated, among which is the classical Boyden chamber assay (transwell assay). In the Boyden chamber assay, cells migrate from the upper to the lower side of a porous membrane, through a gradient of chemoattractant. This assay is valuable for adherent and non-adherent cells, and it offers 3D migration conditions. But its major limitation is that cells can’t be studied while migrating, as with live-cell microscopy. The end results of chemotactic migration in the Boyden chamber are deduced after cell fixation and staining (Chen, 2012). With the development of time-lapse microscopy, based on phase contrast that causes less photodamage, experiments of live-cell imaging for longer periods have become easier to perform. Thus, many 2D random migration devices (Gau and Roy, 2020), and 2D chemotaxis migration devices have been generated (Taylor et al., 2018), such as the ibidi μ-Slide Chemotaxis® (Zengel et al., 2011). One of the first, and efficient 2D chemotaxis migration assays is the Dunn chemotaxis chamber assay (Zicha et al., 1991). It is a glass chamber, with an inner well free of chemoattractant, and an outer well that contains chemoattractant. Hence, a gradient of chemoattractant is formed on the annular bridge between the two wells. Cells are directly observed while migrating on this bridge, using a time-lapse microscope. A detailed description of the assay is available elsewhere (Monypenny et al., 2009; Chaubey et al., 2011).
Dunn chamber assembly is a multistep process that requires good manual dexterity (Taylor et al., 2018). This might be the reason why it is not frequently used. In this protocol, we will explain how to use the Dunn chamber and how to analyse its results, step by step. The essential contribution of this protocol is that we tried to simplify some steps, in assembly and in analysis, and make them user-friendly, to facilitate the use this device. Finally, we used T47D cells in this protocol (Benseddik et al., 2013). However, any adherent cell line could be used with its respective chemoattractant. Plus, cell morphology and polarity could also be closely observed and analysed within this assay.
Materials and Reagents
Hard non curling glass coverslips, No. 3 or No. 2 (Ted Pella, catalog number: 260156)
35 mm culture dishes (Corning, Costar, catalog number: CLS430588)
15 mL conical centrifuge tubes (BD, catalog number: 14-959-49D)
Sterile filter paper (Thermo Fisher Scientific, catalog number: 09-802-1B)
2.5 cm conventional injection needle (Thermo Fisher Scientific, catalog number: 14-817-144)
T47D human breast carcinoma cells (ATCC®, number HTB-133TM)
Dulbecco's modified eagle medium DMEM (Lonza, BioWhittaker, catalog number: BW12-604F)
Heat-inactivated FBS (Corning, catalog number: 35011CV)
100× Antibiotic Penicillin-Streptomycin (Gibco, catalog number: 15140148) (to prevent bacterial and fungal contamination of cell culture)
100× Sodium pyruvate (Gibco, catalog number: 11360-070)
Collagen I, 3 mg/mL (Gibco, catalog number: A1048301)
Phosphate buffered saline (PBS) (Lonza, BioWhittaker, catalog number: BW17516F)
Trypsin-EDTA (Lonza, Trypsin-Versene, catalog number: BW17161E)
Heregulin β1 (HRGβ1) (R&D Systems, catalog number: 396-HB)
Trypan Blue Solution 0.4% (Gibco, catalog number: 15250061)
Dunn chamber (Hawksley, catalog number: DCC100)
DMEM S+ media (see Recipes)
Collagen solution (10 μg/mL) (see Recipes)
Equipment
Flat tweezer (Thermo Fisher Scientific, catalog number: 12342158)
Cell culture incubator set to 37°C and 5% CO2 (Thermo Fisher Scientific, catalog number: 51030303)
Waterbath set to 37°C (Thermo Fisher Scientific, catalog number: TSCIR35)
Class II Type A2 Biological Safety Cabinet (cell culture hood)
Counting chamber Malassez (Marienfeld Superior, catalog number: 0640610)
Motorized Inverted microscope Axiovert 200 M (Carl Zeiss)
Live-cell imaging environmental chamber with 5% (v/v) CO2 in air at 37°C (Climabox, Carl Zeiss)
Camera for microscope (Coolsnap HQ; Roper Scientific®)
10× objective [10× plan apochromat (NA 1.4) objective; Carl Zeiss]
Software
MetaMorph Microscopy Automation and Image Analysis Software® (Molecular Devices)
Chemotaxis and migration tool 1.01, ImageJ Plugin® (ibidi)
ImageJ® (National Institutes of Health)
Microsoft Excel (Microsoft®)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Benseddik, K. and Zaoui, K. (2022). Studying Chemotactic Migration in Dunn Chamber: An Example Applied to Adherent Cancer Cells. Bio-protocol 12(3): e4316. DOI: 10.21769/BioProtoc.4316.
分类
癌症生物学 > 侵袭和转移 > 细胞生物学试验 > 细胞侵袭
癌症生物学 > 侵袭和转移 > 细胞生物学试验 > 细胞迁移
细胞生物学 > 细胞运动 > 细胞运动性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link