- EN - English
- CN - 中文
Cytopathic Effect Assay and Plaque Assay to Evaluate in vitro Activity of Antiviral Compounds Against Human Coronaviruses 229E, OC43, and NL63
细胞病变效应试验和空斑试验评估抗病毒化合物对人冠状病毒229E、OC43和NL63的体外活性
发布: 2022年02月05日第12卷第3期 DOI: 10.21769/BioProtoc.4314 浏览次数: 7576
评审: Juan Facundo Rodriguez AyalaJean Kaoru MilletXinlei LiYolanda Yue Huang
Abstract
Coronaviruses are important human pathogens, among which the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent for the COVID-19 pandemic. To combat the SARS-CoV-2 pandemic, there is a pressing need for antivirals, especially broad-spectrum antivirals that are active against all seven human coronaviruses (HCoVs). For this reason, we are interested in developing antiviral assays to expedite the drug discovery process. Here, we provide the detailed protocol for the cytopathic effect (CPE) assay and the plaque assay for human coronaviruses 229E (HCoV-229E), HCoV-OC43, and HCoV-NL63, to identify novel antivirals against HCoVs. Neutral red was used in the CPE assay, as it is relatively inexpensive and more sensitive than other reagents. Multiple parameters including multiplicity of infection, incubation time and temperature, and staining conditions have been optimized for CPE and plaque assays for HCoV-229E in MRC-5, Huh-7, and RD cell lines; HCoV-OC43 in RD, MRC-5, and BSC-1 cell lines, and HCoV-NL63 in Vero E6, Huh-7, MRC-5, and RD cell lines. Both CPE and plaque assays have been calibrated with the positive control compounds remdesivir and GC-376. Both CPE and plaque assays have high sensitivity, excellent reproducibility, and are cost-effective. The protocols described herein can be used as surrogate assays in the biosafety level 2 facility to identify entry inhibitors and protease inhibitors for SARS-CoV-2, as HCoV-NL63 also uses ACE2 as the receptor for cell entry, and the main proteases of HCoV-OC43 and SARS-CoV-2 are highly conserved. In addition, these assays can also be used as secondary assays to profile the broad-spectrum antiviral activity of existing SARS-CoV-2 drug candidates.
Background
Humans have been battling viruses throughout history. Viral epidemics and pandemics have caused devastating economic, social, and political unrest, in addition to significant morbidity and mortality (Shang et al., 2021). There are seven human coronaviruses: SARS-CoV (Gagneur et al., 2002), MERS-CoV (Zumla et al., 2015), and SARS-CoV-2 (Hui et al., 2020), which cause severe acute respiratory syndrome; and four common human coronaviruses HCoV-229E, HCoV-NL63, HCoV-OC43, and HCoV-HKU1, which cause a significant portion of upper and lower respiratory tract infections in humans worldwide (Mesel-Lemoine et al., 2012). Although vaccines are the mainstay for viral prophylaxis, antiviral drugs are essential complements and play a vital role in viral disease containment, especially during the lag period between the initial viral outbreak and the delivery of effective vaccines. Furthermore, vaccines may lose potency and become ineffective when mutations emerge among circulating viruses (Collier et al., 2021; Lopez Bernal et al., 2021; Williams and Burgers, 2021). As the third coronavirus outbreak in human history, the COVID-19 pandemic is a timely reminder of the urgent need for broad-spectrum antiviral drugs that can be rapidly deployed for the prevention and treatment of emerging and re-emerging viral diseases.
We have routinely used the CPE and plaque assays to test antivirals against many different types of viruses, including influenza, enterovirus, and coronavirus (Hu et al., 2017a, 2017b, 2017c, 2017d, 2018, 2021a, 2021b; Li, F. et al., 2016, 2017; Ma et al., 2019, 2020a, 2020b; Musharrafieh et al., 2019a, 2019b, 2020; Smail et al., 2021; Wang et al., 2018; Xia et al., 2021; Zhang et al., 2018, 2019, 2020). The CPE and plaque assays have several key advantages over alternative methods. First, these assays measure viral infectivity instead of a given viral protein or gene, as both assays directly report the potency of testing compounds in inhibiting the replication of infectious viruses. Second, CPE and plaque assays are more reproducible than the TCID50 assay (Nadgir et al., 2013). Third, both CPE and plaque assays are relatively inexpensive compared to RT-qPCR (Corman et al., 2020), immunoassays (Liu et al., 2005; Payne et al., 2006), viral flow cytometry (Brussaard et al., 2000), or Transmission Electron Microscope (TEM) (Roingeard, 2008), and do not require expensive reagents or specialized instruments. In this study, we have established two cell-based antiviral assays, the CPE assay and the plaque assay for HCoV-229E, HCoV-NL63, and HCoV-OC43. These assays can be used as alternatives for the primary screening of SARS-CoV-2 antivirals in BSL-2 facilities, and also as secondary assays to characterize the broad-spectrum antiviral activity of potential drug candidates. The CPE assay is appropriate for high-throughput screenings in a 96-well plate format, while the plaque assay is comparatively labor-intense and time-consuming, and is more suitable as a secondary assay to confirm the antiviral activity of initial hits identified from the primary CPE assay (Ratnam et al., 1995). Overall, these assays are expected to expedite the discovery of new antivirals against coronaviruses in BSL-2 facilities. In this report, we describe the detailed protocols for utilizing the CPE assay (flowchart illustrated in Figure 1) and the plaque assay (flowchart illustrated in Figure 2) to test antiviral activity of drug candidates against HCoV-229E, HCoV-NL63, and HCoV-OC43. Multiple parameters for the assays have been optimized, including the cell lines, multiplicity of infection, incubation time and temperature, and staining conditions. The optimized CPE and plaque assays conditions for HCoV-229E, HCoV-OC43 and HCoV-NL63 are listed in Table 1. Both assays have been calibrated with the positive control compounds remdesivir (Wang et al., 2020) and GC-376 (Ma et al., 2020c). Alternatively, CPE and plaque assays protocols for HCoV-229E, HCoV-OC43, and HCoV-NL63 can be found elsewhere (Schmidt et al., 1979; Gerna et al., 1980; Herzog et al., 2008; Bracci et al., 2020; Hirose et al., 2021; Schirtzinger et al., 2021).
Table 1. Cell lines and incubation temperature and time for HCoV-229E, HCoV-OC43, and HCoV-NL63 in the CPE and plaque assays in this protocol.
| Virus | Assay | Plate | Cell line | Seeding density; volume per well | Total wells needed for x compounds | Incubation Temperature (°C) | Incubation Time (day) |
| HCoV-OC43 | CPE | 96-well | RD | 2.5×104 cells/mL; 100 µL | 6(x+1)*3 | 33 | 4.5 |
| plaque assay | 6-well | RD | 2.5×104 cells/mL; 3 mL | (6x+1)*2 | 33 | 4.5 | |
| HCoV-229E | CPE | 96-well | MRC-5 | 5×104 cells/mL; 100 µL | 6(x+1)*3 | 33 | 5.5 |
| plaque assay | 6-well | RD | 2.5×104 cells/ml; 3 mL | (6x+1)*2 | 33 | 5.5 | |
| HCoV-NL63 | CPE | 96-well | Vero E6 | 2×104 cells/mL; 100 µL | 6(x+1)*3 | 37 | 4 |
| plaque assay | 6-well | Vero E6 | 2×104 cells/mL; 3 mL | (6x+1)*2 | 37 | 4 |
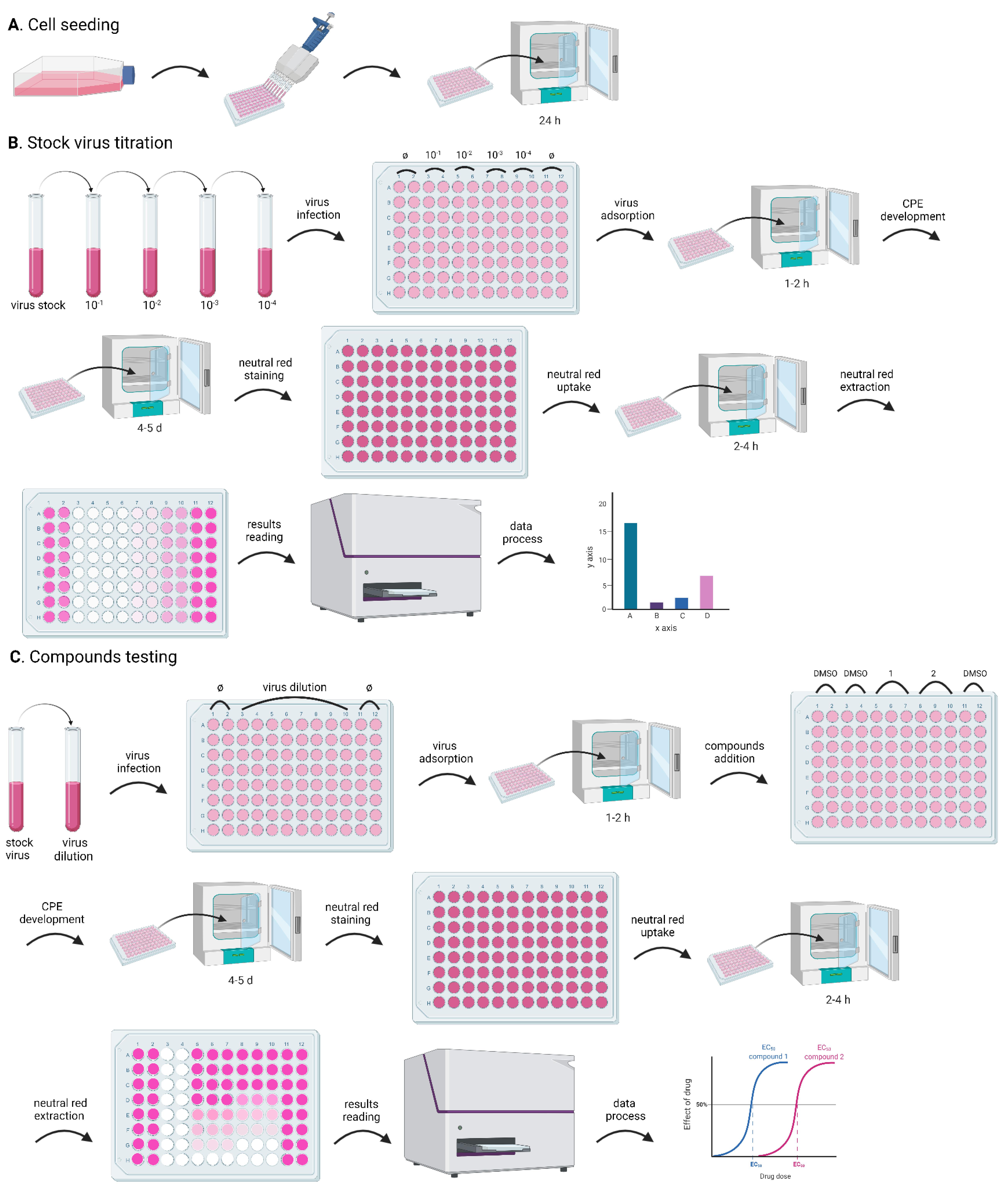
Figure 1. Flowchart for the CPE assay. The whole process includes: cell seeding, to prepare cells in a 96-well plate for the experiments; stock virus titration, to optimize the assay conditions; and compound testing, for the evaluation of the antiviral activity of test compounds. Both stock virus titration and compound testing comprise the following steps: dilute the stock virus to obtain the desired MOI; infect the cells in the 96-well plate with a small volume (100 µL) of the diluted virus; incubate the 96-well plate in the incubator for 1-2 h, to facilitate viral attachment (virus adsorption); add the test compounds (only for the compound testing experiment); incubate the 96-well plate in the incubator to develop CPE; stain the cells with neutral red; extract neutral red from the cells; quantify neutral red by measuring absorbance; analyze data. A. Preparation of cells in the 96-well plate for the CPE assay; B. Major steps of stock virus titration in the CPE assay; C. Assessment of antiviral activity of test compounds in the CPE assay.

Figure 2. Flowchart for the plaque assay. The whole process includes: cell seeding, to prepare cells in a 6-well plate for the experiments; stock virus titration, to optimize the assay conditions; and compound testing, for the evaluation of the antiviral activity of test compounds. Both stock virus titration and compound testing comprise the following steps: dilute the stock virus, to obtain the desired MOI; infect the cells in the 6-well plate with a small volume (500 µL) of the diluted virus; incubate the 6-well plate in the incubator for 1-2 h, to facilitate viral attachment (virus adsorption); add an avicel overlay containing the test compounds (only for the compound testing experiment); incubate the 6-well plate in the incubator, to allow plaque formation; stain the cells with crystal violet; analyze data. A. Preparation of cells in the 6-well plate for the plaque assay; B. Major steps of stock virus titration in the plaque assay; C. Assessment of antiviral activity of test compounds in the plaque assay.
Materials and Reagents
Flat-bottomed 96-well tissue culture plates (Genesee, catalog number: 25-109)
96-well storage plate (Thermo Scientific, catalog number: AB-1058)
96-well deep well storage plates (ThermoFisher Scientific, catalog number: 260251)
50 mL conical centrifuge tubes, sterile, polypropylene (Genesee, catalog number: 25-108)
15 mL conical centrifuge tubes, sterile, polypropylene (Genesee, catalog number: 25-106)
10 mL serological pipets, sterile (Genesee, catalog number: 25-104)
25 mL serological pipets, sterile (Genesee, catalog number: 25-106)
1.7 mL DNase/RNase-free tubes (Genesee, catalog number: 25-282)
1,000 µL pipette tips, low binding (Genesee, catalog number: 24-160R)
200 µL pipette tips, low binding (Genesee, catalog number: 24-150R)
10 µL pipette tips, low binding (Genesee, catalog number: 24-121R)
Tissue culture treated flasks, 600 mL, vent (Genesee, catalog number: 25-211)
Tissue culture treated flasks, 250 mL, vent (Genesee, catalog number: 25-209)
Sterile syringe filter, 0.2 µm (Fisher Scientific, catalog number: 09-740-100)
6-well cell culture plates (Genesee, catalog number: 25-105)
HCoV-OC43 virus (BEI Resources, catalog number: NR-52725)
HCoV-229E virus (BEI Resources, catalog number: NR-52726)
HCoV-NL63 virus (BEI Resources, catalog number: NR-470)
Human rhabdomyosarcoma cell line, RD (ATCC® CCL-136TM)
Human fibroblast cell line, MRC-5 (ATCC® CCL-171TM)
HEK-293T-hACE2 cell line (BEI Resources, catalog number: NR-52511)
A549-hACE2 cell line (BEI Resources, catalog number: NR-53821)
Vero E6 cell line (ATCC® CRL-1586TM)
HCT-8 cell line (ATCC® CCL-244TM)
Huh-7 cell line (Millipore Sigma, catalog number: 01042712-1VL, a kind gift from Dr. Tianyi Wang at the University of Pittsburgh)
BHK-21 cell line (ATCC® CCL-10TM)
BSC-1 cell line (ATCC® CCL-26TM, a kind gift from Dr. Kui Li at the University of Tennessee Health Science Center)
Calu-3 cell line (ATCC® HTB-55TM)
Caco-2 cell line (ATCC® HTB-37TM)
Trypsin-EDTA, 0.25% 1×, phenol red (Genesee, catalog number: 25-510)
Dulbecco's modified Eagle medium (DMEM) (Genesee, catalog number: 25-501)
Eagle's minimum essential medium (EMEM) (ATCC® 30-2003TM)
FBS (Gibco, catalog number: 26140-095)
Penicillin-Streptomycin (P/S) 100× solution (Genesee, catalog number: 25-512)
Glacial acetic acid (CAMEO chemicals, catalog number: UN2789)
Na2HPO4·2H2O (Sigma, catalog number: 04272-1KG)
KH2PO4 (Sigma, catalog number: P9791-1KG)
NaCl (Fisher Scientific, catalog number: BP358-10)
KCl (Sigma, catalog number: P3911-1KG)
Ethanol (ThermoFisher, catalog number: A405-20)
MgCl2·6H2O (Sigma, catalog number: M2670-1KG)
CaCl2 (Sigma, catalog number: C1016-2.5KG)
DMEM powder (Gibco, catalog number: 12-800-017)
HEPES, sodium salt (Gold Biotechnology, catalog number: H-401-2.5)
NaHCO3 (Millipore Sigma, catalog number: S5761-5KG)
Condensed HCl, 36.5 to 38% (Fisher Scientific, catalog number: A144-212)
Avicel microcrystalline cellulose (FMC BioPolymer, Philadelphia, PA)
Crystal violet (Fisher Scientific, catalog number: AAB2193236)
Nano pure, or distilled and deionized water
Phosphate Buffered Saline (PBS) (see Recipes)
Neutral red (3-amino-7-dimethylamino-2-methyl-phenazine hydrochloride) (Sigma, catalog number: N4638)
10× PBS stock (see Recipes)
100× MgCl2 stock (see Recipes)
100× CaCl2 stock (see Recipes)
DPBS, calcium and magnesium free (see Recipes)
PBS, with calcium and magnesium (see Recipes)
Neutral red stock solution (see Recipes)
Complete culture medium (see Recipes)
Neutral red working solution (see Recipes)
Neutral red de-staining solution (see Recipes)
2× DMEM (see Recipes)
1.2% avicel (see Recipes)
0.2% crystal violet (see Recipes)
Equipment
Two cell culture incubators (Eppendorf, Galaxy® 170 R), humidified, 5% CO2/95% air, with temperatures set up at 33°C and 37°C, respectively
Inverted microscope Olympus CKX53 (ThermoFisher Scientific, catalog number: NC1991101)
MultiskanTM FC Microplate Photometer (ThermoFisher Scientific, catalog number: 51119000)
CountessTM 3 Automated Cell counter (ThermoFisher Scientific, catalog number: AMQAX2000)
Fume hood (for hazardous chemicals)
Microtiter plate shaker (Fisher Scientific, catalog number: 88-861-023)
Hot plate stirrer (Fisher Scientific, catalog number: 11-520-49SH)
PIPETMAN Classic P10 (Genesee, catalog number: 37-100P10)
PIPETMAN Classic P100 (Genesee, catalog number: 37-100P100)
PIPETMAN Classic P1000 (Genesee, catalog number: 37-100P1K)
PIPETMAN Classic P20 (Genesee, catalog number: 37-100P20)
PIPETMAN Classic P200 (Genesee, catalog number: 37-100P200)
Reagent reservoir, sterile (VWR, catalog number: 89094-662)
Vortex Mixer (Fisher Scientific, catalog number: 02-215-414)
Centrifuge (Beckman, catalog number: A99465)
Bottle Top Vacuum Filters (Genesee, catalog number: 25-235)
SartoriusTM BiohitTM PicusTM NxT Electronic Pipettes, 50-1,200 µL, 12 Channels (SartoriusTM, catalog number: LH745491)
SartoriusTM BiohitTM PicusTM NxT Electronic Pipettes, 0.2-10 µL, 12 Channels (SartoriusTM, catalog number: LH745421)
HandE-Vac Handheld Aspirating System (Argos TechnologiesTM, catalog number: 10-987-042)
Software
ImageJ (National Institutes of Health, version 1.50 c, https://imagej.nih.gov/ij/download.html)
Prism GraphPad (https://www.graphpad.com)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Hu, Y., Ma, C. and Wang, J. (2022). Cytopathic Effect Assay and Plaque Assay to Evaluate in vitro Activity of Antiviral Compounds Against Human Coronaviruses 229E, OC43, and NL63. Bio-protocol 12(3): e4314. DOI: 10.21769/BioProtoc.4314.
分类
微生物学 > 抗微生物试验 > 抗病毒试验
药物发现 > 药物筛选
生物化学 > 病毒 > 分离和纯化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link