- EN - English
- CN - 中文
Developing Clinically Relevant Acquired Chemoresistance Models in Epithelial Ovarian Cancer Cell Lines
上皮性卵巢癌细胞临床相关获得性化疗耐药模型的建立
(*contributed equally to this work) 发布: 2022年02月05日第12卷第3期 DOI: 10.21769/BioProtoc.4310 浏览次数: 4210
评审: Giusy TornilloVishal NehruKristina AguileraTalita Diniz Melo HanchukManoj B. Menon
Abstract
Chemoresistance, the ability of cancer cells to overcome therapeutic interventions, is an area of active research. Studies on intrinsic and acquired chemoresistance have partly succeeded in elucidating some of the molecular mechanisms in this elusive phenomenon. Hence, drug-resistant cellular models are routinely developed and used to mimic the clinical scenario in-vitro. In an attempt to identify the underlying molecular mechanisms that allow ovarian cancer cells to gradually acquire chemoresistance, we have developed isogenic cellular models of cisplatin and paclitaxel resistance (singularly and in combination) over six months, using a clinically relevant modified pulse method. These models serve as important tools to investigate the underlying molecular players, modulation in genetics, epigenetics, and relevant signaling pathways, as well as to understand the role of drug detoxification and drug influx-efflux pathways in development of resistance. These models can also be used as screening tools for new therapeutic molecules. Additionally, repurposing therapeutic agents approved for diseases other than cancer have gained significant attention in improving cancer therapy. To investigate the effect of metformin on acquirement of chemoresistance, we have also developed a combinatorial model of metformin and platinum-taxol, using two different strategies. All these models were subsequently used to study modulation in receptor tyrosine kinase pathways, cancer stem cell functionalities, autophagy, metastasis, metabolic signatures, and various biological processes during development of chemoresistance. Herein, we outline the protocols used for developing these intricate resistant cellular models.
Graphic abstract:
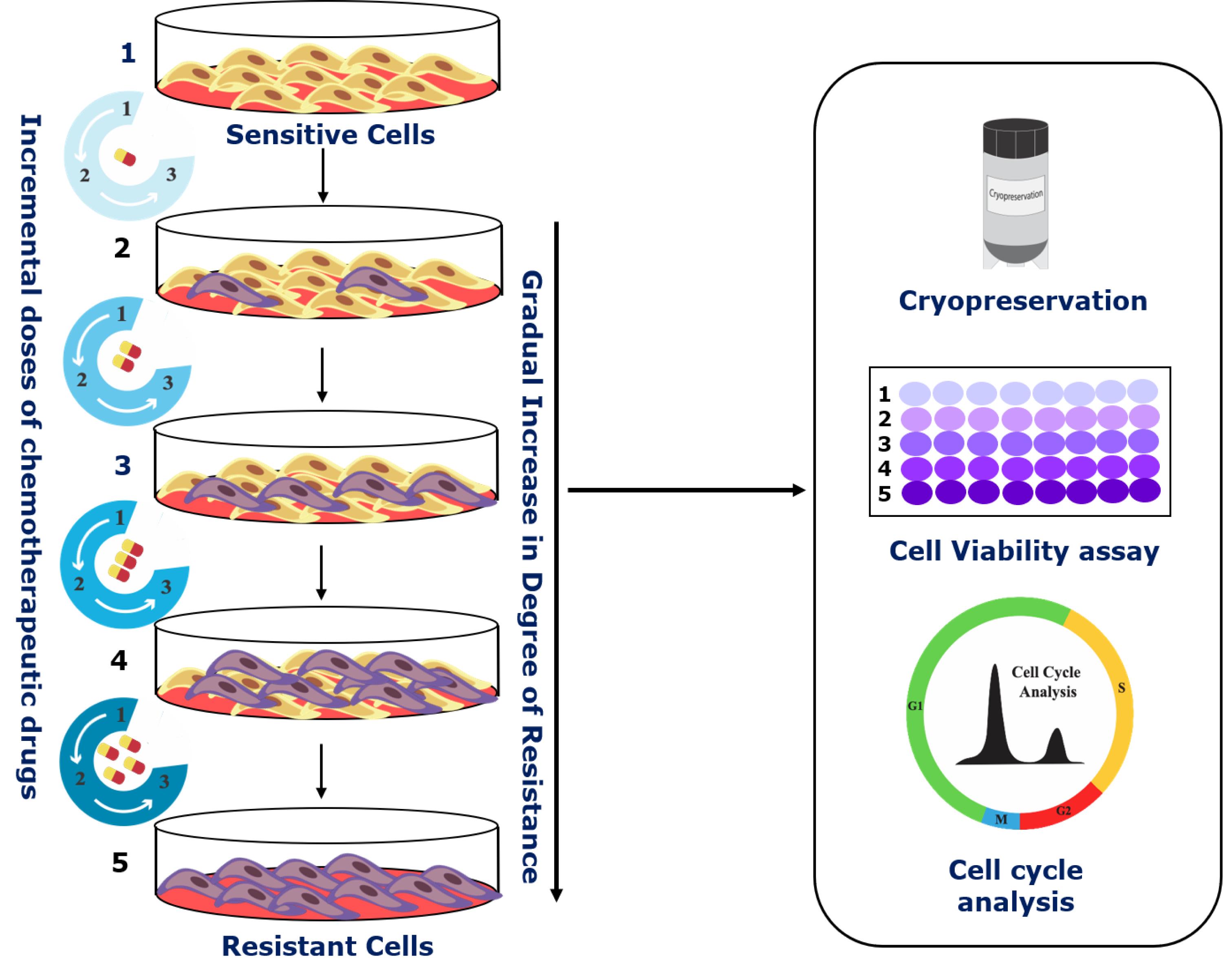
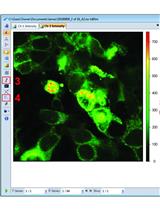
Schematic of the step-wise development of cellular chemoresistant model. Schema illustrating the modified pulse method for the development of individual and combinatorial resistant models. Two different cell lines (A2780 and OAW42) were exposed to drug treatment (either alone or in various combinations) of one concentration for three consecutive cycles (3×). Furthermore, the surviving cells were sub-cultured subsequently with increasing drug concentrations. After every treatment cycle, 50% of the cells were cryo-preserved for further experiments. Additionally, to check for the development of chemoresistance and to assess the changes in cell cycle during resistance development, cell viability assays and flow cytometry were performed.
Background
Chemoresistance is described as the ability of cancer cells to circumvent or soldier through therapeutic interventions. Studies have established the existence of a certain population of tumor cells amongst an otherwise heterogeneous milieu of cells that harbor intrinsic or gradually acquire a chemoresistance phenotype. This population of cells is considered to be a potent challenge in oncology research (Zheng, 2017). Furthermore, cases of relapse, cancer dissemination, and subsequent death can directly or indirectly be linked to chemoresistance (Yeldag et al., 2018). Even though ongoing research has improved our knowledge regarding chemoresistance, better systemic and integrative models need to be developed, to tackle this complication head-on.
Considered the deadliest amongst gynecological malignancies, identifying novel targets to combat chemoresistance is currently a significant hurdle in ovarian cancer treatment. Many molecular mechanisms have been attributed to developing resistance against the cytotoxic effects of common chemotherapeutic drugs, such as cisplatin and paclitaxel. These mechanisms comprise a diverse set of complex and often intermingling cell signaling machinery such as the PI3K/Akt/mTOR pathway (Deng et al., 2019) or Wnt/β-catenin signaling (Nguyen et al., 2019), which are deemed essential for acquiring resistance. These pathways are multifactorial and poorly understood, making their targeting ineffective. Cell lines established from EOC patients are routinely utilized to investigate the alteration in these molecular pathways post drug treatments, as well as during development of resistance.
In a clinical setting, the cells gradually begin acquiring resistance to the ongoing course of treatment by expressing specific signaling molecules. These signaling molecules allow the cells to better adapt to the external environment, thereby allowing them to survive and proliferate further. Hence, developing appropriate drug-resistant cellular models to mimic this scenario is essential to understand and discover the signaling cascades associated with chemoresistance regulation (Yan et al., 2007). Drug-resistant cell models are broadly classified into conventional and clinically relevant models. The conventional method involves incremental administration of drugs at low doses with every cycle, whereas the clinically relevant model employs a pulsed treatment, wherein the cells are allowed to grow after every treatment cycle. However, both these traditionally accepted methods have a fair share of advantages and disadvantages. The conventional method creates highly resistant cells, but the heterogeneity observed in these models makes it difficult to identify the underlying molecular players responsible for chemoresistance. Moreover, it is not clinically relevant. The clinically relevant model successfully mimics the treatment cycles administered to a patient in the clinic, but it creates low-level resistance with significantly few molecular changes, thereby making it hard to detect and study. Establishing clinically relevant drug-resistant cell lines involves treating the cells with a fixed drug dosage consistently. This is called the pulse method, and it better encapsulates the biological transition in patients of an otherwise chemo-sensitive cell into gaining chemoresistance. Additionally, the repurposing of existing non-oncology drugs has also emerged as an effective adjuvant in complementing conventional chemotherapeutic regimens (Zhang et al., 2020).
Our laboratory employs a modified version of the pulse method, wherein the parent cells are treated with multiple cycles of incremental drug doses. This leads to the development of a set of isogenic ovarian cancer cell lines resistant to cisplatin, paclitaxel, or both. Furthermore, we also checked the effect of metformin, used for type 2 diabetes treatment, on acquiring chemoresistance in combination with platinum-taxol. An increase in drug concentration after every few treatment cycles is instrumental in the selection and proliferation of only the surviving cells. The isogenic chemoresistant cells obtained from the modified pulse method reflect the clinical scenario and show high resistance indices. Additionally, they also allow the investigator to accurately identify the key molecular players responsible for attaining this chemoresistant phenotype. Though we majorly narrated our methods for A2780 and OAW42 cell lines, these methods can be adapted for any cell line of various cancer origins.
Materials and Reagents
96-well plate (Himedia, catalog number: TPP96)
Cell lines used: A780 and OAW42 cell lines (Passage 10-15) were obtained from Late Dr. Sam Gambhir’s lab, Radiology department of Stanford University, USA (STR profiling of the cells have been included in the Supplementary File).
DMEM (Gibco/Invitrogen, catalog number: 12800017)
Fetal Bovine Serum (FBS) (Himedia, catalog number: RM10409)
Penicillin-Streptomycin (Himedia, catalog number: A002)
Trypsin-EDTA (Gibco/Invitrogen, catalog number: 25200072)
DMSO (Himedia, catalog number: TC185)
Cisplatin (Sigma, catalog number: P4394)
Paclitaxel (Sigma, catalog number: T1912)
Metformin (Sigma, catalog number: PHR1084)
Superfect transfection reagent (Qiagen, catalog number: 301307)
Thiazolyl Blue Tetrazolium Bromide (Sigma, catalog number: M2128-1G)
Propidium Iodide (Sigma, catalog number: P4170)
RNase A (Sigma, catalog number: R4875)
Drug stocks (see Recipes)
Equipment
Benchtop Centrifuge (Eppendorf Centrifuge, model: 5804 R)
Incubator (Thermo Scientific, model: 3121)
Esco Class II Biological Safety Cabinet
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Shenoy, P. S., Chakraborty, S., Gaikwad, S. M., Sakpal, A. and Ray, P. (2022). Developing Clinically Relevant Acquired Chemoresistance Models in Epithelial Ovarian Cancer Cell Lines. Bio-protocol 12(3): e4310. DOI: 10.21769/BioProtoc.4310.
分类
癌症生物学 > 癌症生物化学 > 耐药性
细胞生物学 > 细胞分离和培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link