- EN - English
- CN - 中文
HiSAT: A Novel Method for the Rapid Diagnosis of Allergy
HiSAT:一种快速诊断过敏的新方法
发布: 2022年02月05日第12卷第3期 DOI: 10.21769/BioProtoc.4309 浏览次数: 2306
评审: Alessandro DidonnaScott McCombSvetlana Kurilova
Abstract
To identify causative substances for allergies to drugs or foods, the lymphocyte transformation test (LTT) is currently widely used as in vitro test, but its accuracy is not satisfactory. We have developed a novel method designated high-sensitivity allergy test (HiSAT) for determining allergy expression by measuring cell kinetics, using the chemotactic cells from non-allergic volunteers against a gradient field of cytokines released from immune cells when allergy develops. HiSAT requires a very small sample of 5 µL or less, and is applicable to three types of tests, depending on the situation in clinical practice: (i) diagnosis of the allergic expression, (ii) identification of the causative drug, and, in principle, (iii) pre-inspection.
Graphic abstract:
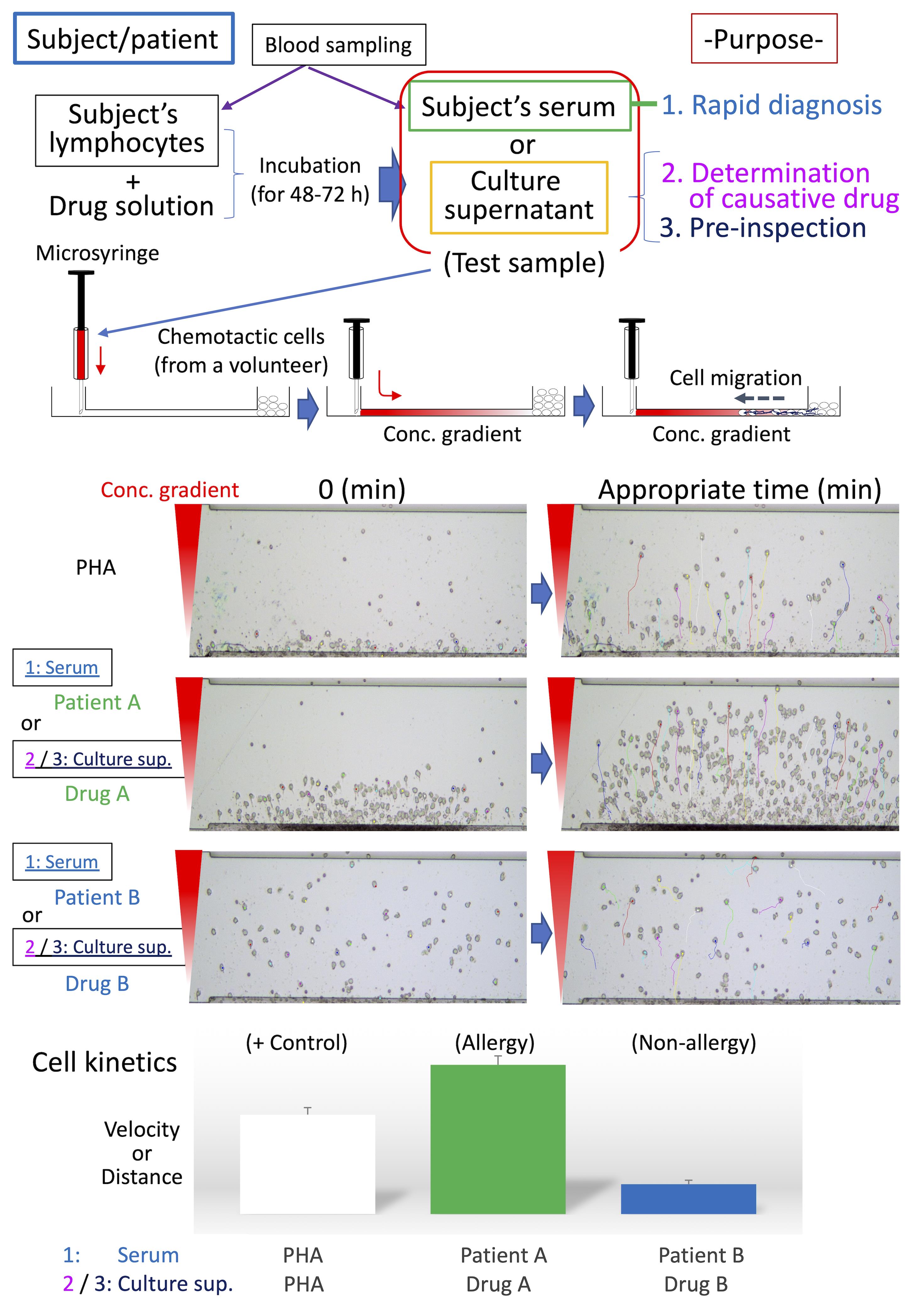
Schematic diagram of HiSAT. Serum from patients/subjects is used for rapid diagnosis in HiSAT. To identify the causative drug, the lymphocytes of interest are incubated with the candidate drug solution for 48 h to 72 h and then the culture supernatant is used in HiSAT. Before drug administration, it may possible to avoid the risk of allergies by performing pre-inspection, as well as the determination of the causative drug in HiSAT. A granulocyte-rich cell layer isolated from a non-allergic volunteer is used in HiSAT. Chemotactic cells migrate toward chemotactic factors in the test sample according to the concentration gradient. Cell kinetics (e.g., velocity or distance) are analyzed using sequential images of the test samples, and compared to the PHA-positive control.>
Background
Allergies, especially drug-induced allergy (DIA) or drug-induced hypersensitivity syndrome (DIHS), are unexpectedly caused by drugs used to treat the underlying disease, resulting in discontinuation of treatment or having to deal with allergic symptoms. Therefore, DIA is a heavy burden for both patients and medical personnel. Although it is strongly required in clinical practice, there is currently no rapid test to diagnose whether a patient's symptoms are allergic. On the other hand, once allergies are expressed in patients, it is necessary to identify the causative medicine. LTT is used as a general in vitro method for identifying the causative drug when DIA develops, and a specific antibody test such as RAST (Radioallergosorbent test) or MAST (Multiple allergosorbent test) is used against food-based or inhaled allergens. However, these tests have issues with accuracy or target limitation (Srinoulprasert, 2021). Furthermore, the conventional in vivo tests, such as the prick test, the scratch test, the intradermal test, and the patch test, lack accuracy in risk prediction. In addition, they might be burdensome on patients in positive cases, because of potentially serious allergic symptoms, such as anaphylactic shock. We have developed HiSAT, a novel allergy test, to diagnose allergy by analyzing cell kinetics of chemotactic cells from a non-allergic volunteer on the EZ-TAXIScan, using the subject's serum or the culture supernatant incubating the subject's lymphocytes and candidate drug (Shibaguchi et al., 2021). The advantages of HiSAT are as follows: (i) the amount of the sample is very small (5 µL or less is required), (ii) rapid diagnosis of about 1 hr or less is possible if using subject serum, (iii) the reactivity of each subject is directly confirmed in vitro, (iv) it is possible to test even new substances that have not been reported so far, (v) it does not depend on the expression mechanism (Warrington et al., 2018) because it targets the humoral components released when allergy develops, and (vi) in principle, it is possible to use for pre-inspection (Figure 1).
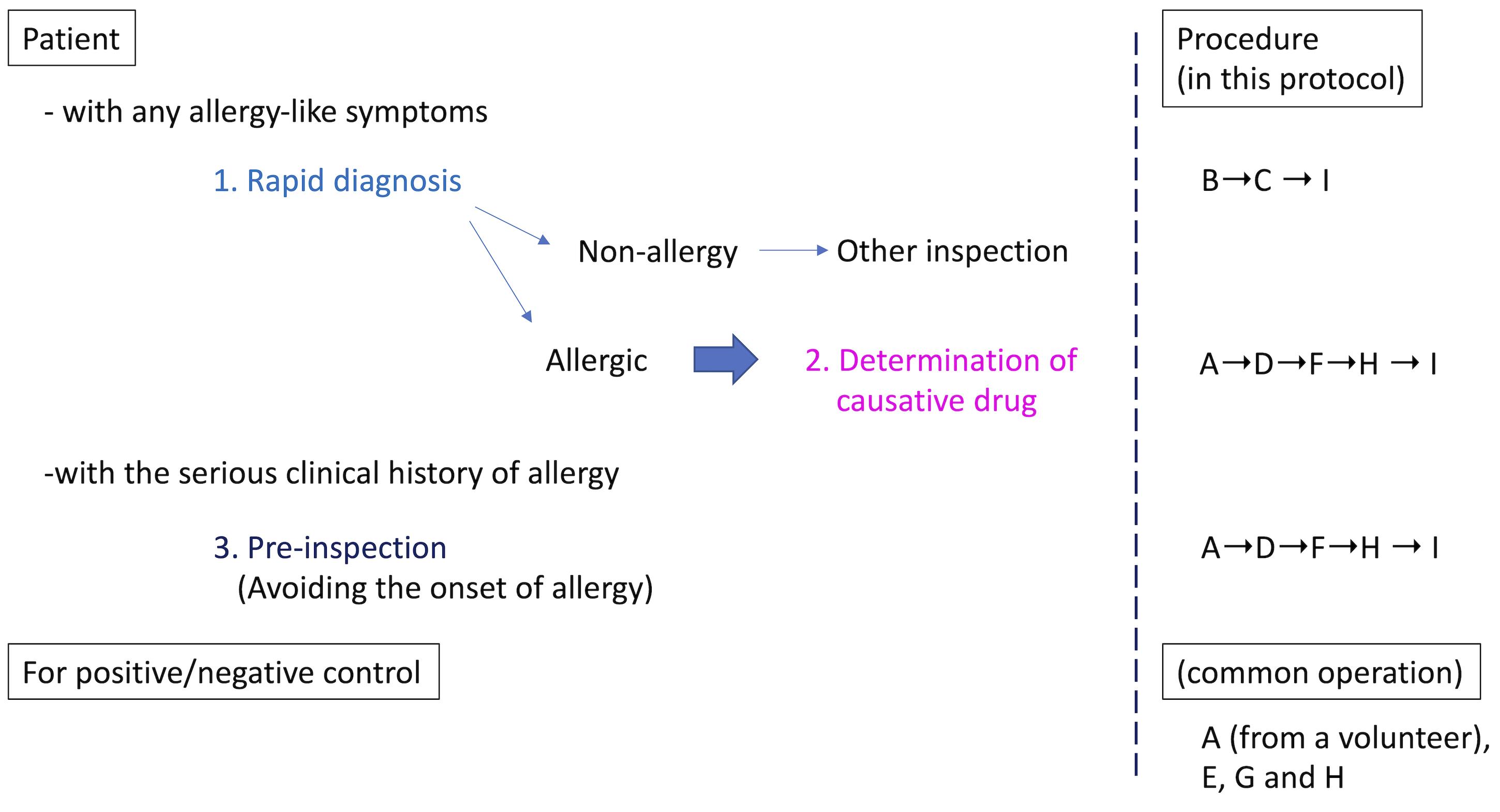
Figure 1. Schematic procedure in HiSAT. If there are any allergy-like symptoms in the patient, the rapid test using the patients' serum is useful. There is no such rapid test so far. Once the symptom is diagnosed as allergic, it is important to determine the causative drug. On the other hand, if a patient has a serious clinical history of allergy, pre-inspection might be useful, to avoid the dangerous drug before use. In all cases, the positive and negative controls have to be prepared in advance.
Materials and Reagents
1-mL syringe (TERMO Co. Ltd., catalog number: SS-01T)
1.5 mL microtube (Funakoshi Co. Ltd., catalog number: 1210-00)
15 mL Falcon tube (Corning, catalog number: 352096)
Pipette tip (P20, P100, P1000: Greiner, catalog numbers: 771351, 770320, 770340)
Pipette (GILSON, M&S Instruments Inc., catalog numbers: F144802, F123601, F123602)
Pasteur pipette (Funakoshi Co. Ltd., cattalog number: 63B92)
10-µL hamilton microsyringe (GL Science, catalog number: 4015-63015)
4 µm thickness of tip for EZ-TAXIScan (GE Healthcare Bioscience Co. Ltd., catalog number: EZT-01-4)
24-well plates (BD Falcon, Corning, catalog number: 353047)
Phosphate buffered saline (PBS, pH 7.4; Thermo Fisher Scientific, Gibco, catalog number: 158127)
RPMI 1640 (pH 7.4, Sigma-Aldrich, catalog number: R8758)
Dalbecco Modified Eagle's Medium (DMEM, pH 7.4, Sigma-Aldrich, catalog number: D6429)
Horse serum (Thermo Fisher Scientific, Gibco, catalog number: 26050070)
Fetal bovine serum (FBS; Fujifilm Wako Chemicals Co.,Ltd., catalog number: FB-1345)
Penicillin (10,000 units) and streptomycin (10 mg) (Sigma-Aldrich, catalog number: P4333)
Venoject II (Terumo Coop., catalog number: VP-AS109K)
Phytohemagglutinin (PHA; Fujifilm Wako Chemicals Co.,Ltd., catalog number: 161-15251)
Lymphoprep (Cosmo Bio Co.Ltd., catalog number: 331312)
Lymphocyte-poly Cell Separation Media (Cosmo Bio Co. Ltd., catalog number: CL5070)
Dimethyl sulfoxide (DMSO; Sigma-Aldrich, catalog number: D2650)
Mosapride citrate hydrate (Sumitomo Dainipon Pharma Co. Ltd., KEGG drug: D00724)
Sulpiride (Nichi-iko Pharmaceutical Co. Ltd., KEGG drug: D01226)
Rabeprazole sodium (Eisai Co, Ltd., KEGG drug: D00724)
Equipment
Centrifuge (TOMY Co. Ltd., catalog number: NIX-521)
Incubator (37°C, 5% CO2) (Thermo Fisher Scientific, catalog number: 4130TS)
Refrigerator (NIHON Freezer Co. Ltd., catalog number: KGT-4056HC)
Freezer (NIHON Freezer Co. Ltd., catalog number: VT-208)
Inverted microscope (Olympus Co. Ltd., catalog number: CKX-53)
EZ-TAXIScan system (GE healthcare Bioscience Co. Ltd., catalog number: MIC-1001)
Software
ImageJ software: Image Processing and Analysis in Java, "https://imagej.nih.gov/index.html", National Institutes of Health, Bethesda, MD, USA
Chemotaxis and Migration Tool, "https://ibidi.com/chemotaxis-analysis/171-chemotaxis-and-migration-tool.html", ibidi GmbH, Gräfelfing, Bayern, DE
GraphPad Prism software: "https://www.graphpad.com/scientific-software/prism/", GraphPad software, La Jolla, CA, USA
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Shibaguchi, H. and Yasutaka, Y. (2022). HiSAT: A Novel Method for the Rapid Diagnosis of Allergy. Bio-protocol 12(3): e4309. DOI: 10.21769/BioProtoc.4309.
分类
免疫学 > 炎症性疾病 > 过敏症
医学
细胞生物学 > 细胞运动 > 细胞迁移
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link