- EN - English
- CN - 中文
Investigation of Microvesicle Uptake by Mouse Lung-marginated Monocytes in vitro
小鼠肺边缘单核细胞体外微囊泡摄取
发布: 2022年02月05日第12卷第3期 DOI: 10.21769/BioProtoc.4307 浏览次数: 4148
评审: Gal HaimovichRajesh ThippeshappaKrisztina Vukman
Abstract
Extracellular microvesicles (MVs) are released into the circulation in large numbers during acute systemic inflammation, yet little is known of their intravascular cell/tissue-specific interactions under these conditions. We recently described a dramatic increase in the uptake of intravenously injected MVs by monocytes marginated within the pulmonary vasculature, in a mouse model of low-dose lipopolysaccharide-induced systemic inflammation. To investigate the mechanisms of enhanced MV uptake by monocytes, we developed an in vitro model using in vivo derived monocytes. Although mouse blood is a convenient source, monocyte numbers are too low for in vitro experimentation. In contrast, differentiated bone marrow monocytes are abundant, but they are rapidly mobilized during systemic inflammation, and thus no longer available. Instead, we developed a protocol using marginated monocytes from the pulmonary vasculature as an anatomically relevant and abundant source. Mice are sacrificed by terminal anesthesia, the lungs inflated and perfused via the pulmonary artery. Perfusate cell populations are evaluated by flow cytometry, combined with in vitro generated fluorescently labelled MVs, and incubated in suspension for up to one hour. Washed cells are analyzed by flow cytometry to quantify MV uptake and confocal microscopy to localize MVs within cells (O'Dea et al., 2020). Using this perfusion-based method, substantial numbers of marginated pulmonary vascular monocytes are recovered, allowing multiple in vitro tests to be performed from a single mouse donor. As MV uptake profiles were comparable to those observed in vivo, this method is suitable for physiologically relevant high throughput mechanistic studies on mouse monocytes under in vitro conditions.
Graphic abstract:
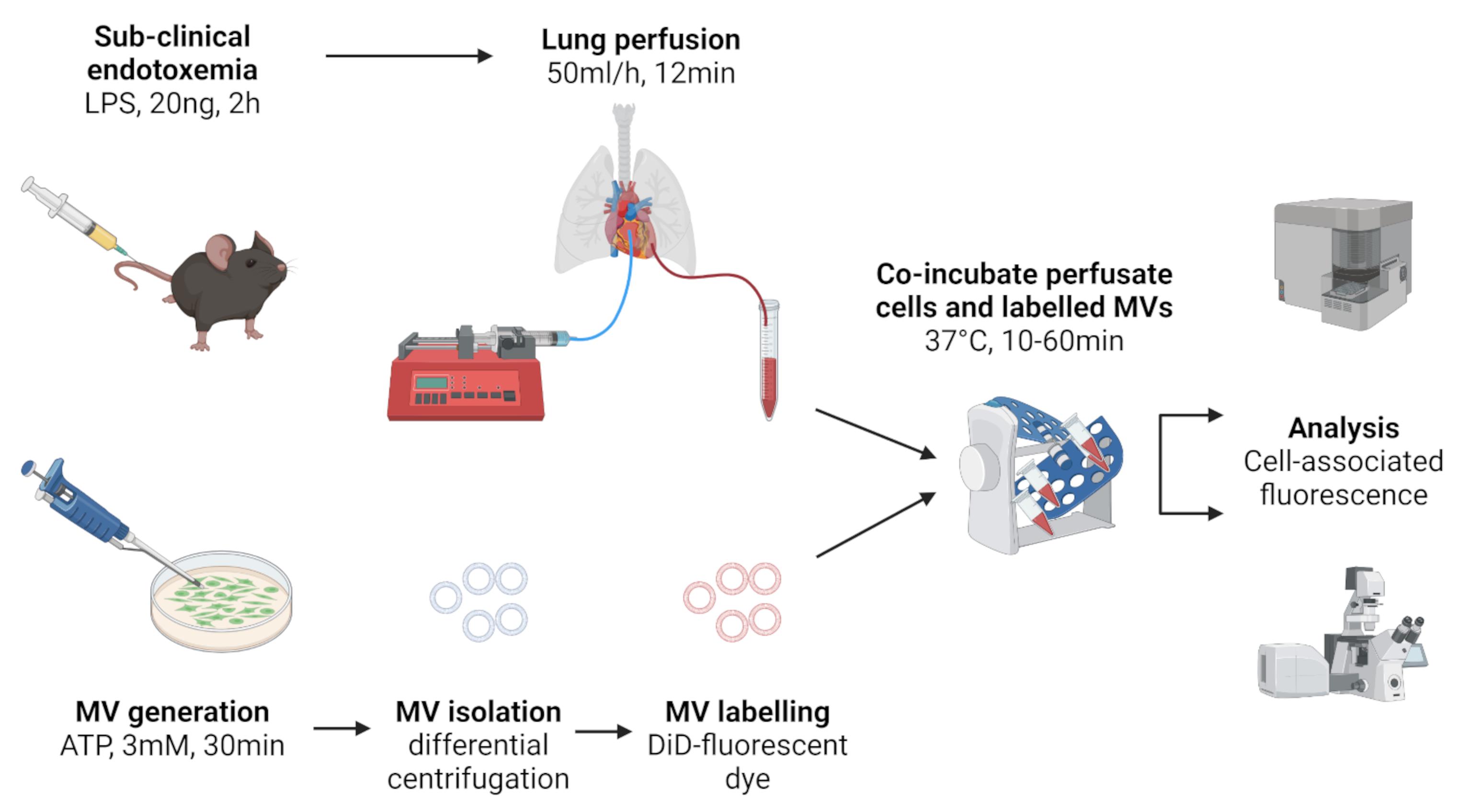
Figure 1. Schematic of lung perfusate cell harvest and co-incubation with in vitro generated MVs. Created with BioRender.com.
Background
Extracellular vesicles (EVs) are a family of cell-derived membrane-encapsulated particles produced constitutively, but also in response to inflammatory stimuli, or activation of programmed cell death pathways (Yanez-Mo et al., 2015). Carrying functional molecular cargoes, EVs have considerable potential as short- and long-range intercellular communication vehicles. Microvesicles (MVs) are an EV subclass derived directly from the cell membrane that display lineage-specific surface markers enabling identification of the parental cell source. During diseases associated with systemic inflammation, such as sepsis and severe burns injury, circulating levels of platelet- and myeloid cell-derived MVs are increased acutely and associated with clinical severity (Nieuwland et al., 2000; Joop et al., 2001; O'Dea et al., 2016). As these MV subtypes carry pro-inflammatory molecular cargoes, they may play a key role in long-range propagation of organ injury during systemic inflammation. Despite this potential, little is known of MV trafficking within the circulation and their cell/tissue-specific interactions during systemic inflammation.
We previously developed a lipopolysaccharide (LPS)-induced model of sub-clinical endotoxemia to evaluate cell/tissue specific interactions of circulating MVs under systemic inflammatory conditions in vivo. In vitro generated MVs labelled with the fluorescent lipophilic dye, 1,1'-dioctadecyl-3,3,3',3'-tetramethylindodicarbocyanine (DiD) were injected intravenously and their cellular uptake quantified in lung, liver, and spleen cell suspensions by flow cytometry (O'Dea et al., 2020). Using in vivo analysis of cell-associated MVs rather than biodistribution methods, which measure total tissue/organ associated MV label (Willekens et al., 2005; Wiklander et al., 2015), enabled us to determine the relative uptake of MVs by different cell populations within and between organs. We found that during systemic inflammation there is a dramatic increase in MV uptake by pulmonary intravascular ‘marginated’ monocytes, resulting in an overall ≥20-fold increase in uptake by the lungs.
To enable more detailed analyses of the enhanced MV-monocyte interactions during systemic inflammation, we developed a novel in vitro model of MV uptake by in vivo derived pulmonary vascular perfusate cells. In vitro analysis of intravascular monocyte functions, including uptake of MVs, is severely constrained in mice due to their low abundance and small volumes of blood; this problem is further exacerbated by withdrawal of monocytes from the circulation by their margination to vascular beds of lungs, liver, and other tissues during systemic inflammation (O'Dea et al., 2005 and 2009; Hamon et al., 2017). Although differentiated monocytes can be obtained from the bone marrow reserve, such preparations are mixed with immature precursors and, moreover, much of the mature monocyte pool is rapidly released into the circulation during inflammation (O'Dea et al., 2009; Shi et al., 2011). Conversely, the pulmonary circulation is a major vascular site for monocyte accumulation, marginated in significant numbers within its vast capillary network under both resting and inflammatory conditions (Doherty and Worthen, 1994; Kuebler and Goetz, 2002). As such, the lungs represent a previously ‘untapped’ source for in vitro evaluation of monocytes and neutrophils, which also accumulate in substantial numbers within the pulmonary circulation during inflammation (van Eeden et al., 1997; Yipp et al., 2017).
Here, we describe in detail our protocol (summarized in Figure 1) for routine recovery of pulmonary vascular cells and evaluation of MV uptake by monocyte subsets and neutrophils, the findings of which were reported recently by us (Figure 6, Figure 7, and Supplementary Figure S2 in O'Dea et al., 2020). In brief, using this method we determined that MV uptake by monocytes was energy-dependent, involved endocytic processes, and could be inhibited in the presence of phosphatidylserine-enriched liposomes, β3 integrin receptor blocking peptide, and scavenger receptor A blockers.
Materials and Reagents
Culture dishes, 60 mm × 15 mm (Corning, tissue-culture treated, catalog number: CLS430166)
PVC OD 0.61 mm × ID 0.28 mm (SteriHealth, catalog number: I-10317)
Polyethylene tubing OD 1.27 mm × ID 0.86 mm (Portex, catalog number: 800/100/260)
15 mL collection tube (Falcon, polypropylene, catalog number: 352096)
1.5 mL microfuge tube (Starlab, catalog number: S1615-5510)
5 mL flow cytometry tube (Falcon, round bottom polypropylene, catalog number: 35200)
96 well black plate (Thermo Scientific, flat bottom non-treated, catalog number: 237108)
27 G needle (VWR, catalog number: 613-3834)
25 G needle (VWR, catalog number: 613-4952)
19 G needle (VWR, catalog number: 613-3938)
1 mL hypodermic syringe (Terumo, catalog number: SS-01T1)
4-0 curved-needle suture (Medisave, catalog number: W586H)
5-0 suture (Covidien, Sofsilk, catalog number: S-182)
2-0 suture (Covidien, Sofsilk, catalog number: S-185)
Straight hemostatic forceps (Fine Science Tools, catalog number: 1301414)
C57/BL6 male mice (Charles River Laboratories, Margate, UK)
J774A.1 macrophage cell line (European Collection of Authenticated Cell Cultures (ECACC), ECACC-91051511)
Lipopolysaccharide (LPS) (InvivoGen, Ultrapure Escherichia coli O111:B4, catalog number: tlrl-3pelps)
Adenosine 5'-triphosphate (ATP) (Bio-Techne, disodium salt, catalog number: 3245)
Heparin sodium (Wockhardt, 1,000 I.U./mL, catalog number: FP1086)
Human Serum Albumin (HSA) (BioIVT, 25% Solution, catalog number: GEM-800-120-S)
Fetal Bovine Serum (FBS) (Sigma, heat inactivated/sterile filtered, catalog number: F9665)
RPMI-1640 medium (Sigma, catalog number: R5886)
Penicillin-streptomycin (Sigma, catalog number: P4333-100mL)
Antibodies for flow cytometry (see Table 1)
Table 1. Fluorophore-conjugated antibodies for flow cytometric analysis of perfusate cells.
Antigen Fluorophore Dilution Clone Company/Catalog no. F4/80 FITC 1:400 BM8 Biolegend/123108 CD11b PE 1:400 M1/70 Biolegend/101208 Ly-6C PE-Cy7 1:400 HK1.4 Biolegend/128018 Ly-6G APC-Cy7 1:400 1A8 Biolegend/127624 Ly-6G/Ly-6C (Gr-1) APC 1:400 RB6-8C5 Biolegend/108412 AccuCheck Counting Beads (ThermoFisher Scientific, catalog number: PCB100)
Vybrant® DiD cell-labeling solution (ThermoFisher Scientific, catalog number: V-22887)
Diluent C (Sigma, catalog number: CGLDIL-6X10mL)
Phosphate buffered saline (PBS) with Ca2+/Mg2+ (Sigma, catalog number: D8662-500mL)
Phosphate buffered saline (PBS) 10× (Fisher scientific, 20 l, catalog number: BP399-20)
Hank’s Buffered Salt Solution (HBSS) without Ca2+/Mg2+ (Sigma, catalog number H6648)
Hank’s Buffered Salt Solution (HBSS) with Ca2+/Mg2+ (Sigma, catalog number: 55037C)
TritonTM X-100 (Sigma, catalog number: X100-500ML)
Ethylenediaminetetraacetic acid (EDTA) (ThermoFisher Scientific, catalog number: 15575020)
Sodium azide (Sigma, catalog number: S2002)
Perfusion buffer (see Recipes)
Assay buffer (see Recipes)
Flow cytometry buffer (see Recipes)
Equipment
Syringe pump (Harvard Apparatus, model: 11/55-1199)
Refrigerated microfuge (Eppendorf, model: 5417R; angle rotor: FA45-30-11)
Refrigerated benchtop centrifuge (Heraeus, model: Multifuge 3S-R)
Fluorescence plate reader (BioTek, model: Flx-800)
Flow cytometer (Beckman-Coulter, model: CyAn ADP)
Blood Test Tube Rotator (Stuart Scientific, model: SB1)
Incubator (Memmert, model: IN55)
Software
KC4 data analysis software (BioTek, https://www.biotek.com/)
FlowJoTM Software Version 10.5.3 (Ashland, OR, https://www.flowjo.com/)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Tan, Y. Y., O'Dea, K. P., Patel, B. V. and Takata, M. (2022). Investigation of Microvesicle Uptake by Mouse Lung-marginated Monocytes in vitro. Bio-protocol 12(3): e4307. DOI: 10.21769/BioProtoc.4307.
分类
免疫学 > 免疫细胞分离 > 骨髓细胞
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link