- EN - English
- CN - 中文
From 3D to 2D: Harmonization of Protocols for Two-dimensional Cultures on Cell Culture Inserts of Intestinal Organoids from Various Species
从三维到二维:不同物种肠道类器官细胞培养插件的二维培养方法的协调
(*contributed equally to this work) 发布: 2022年01月20日第12卷第2期 DOI: 10.21769/BioProtoc.4295 浏览次数: 5833
评审: Giusy TornilloJens PuschhofTakashi Nishina
Abstract
In the expanding field of intestinal organoid research, various protocols for three- and two-dimensional organoid-derived cell cultures exist. Two-dimensional organoid-derived monolayers are used to overcome some limitations of three-dimensional organoid cultures. They are increasingly used also in infection research, to study physiological processes and tissue barrier functions, where easy experimental access of pathogens to the luminal and/or basolateral cell surface is required. This has resulted in an increasing number of publications reporting different protocols and media compositions for organoid manipulation, precluding direct comparisons of research outcomes in some cases. With this in mind, here we describe a protocol aimed at the harmonization of seeding conditions for three-dimensional intestinal organoids of four commonly used research species onto cell culture inserts, to create organoid-derived monolayers that form electrophysiologically tight epithelial barriers. We give an in-depth description of media compositions and culture conditions for creating these monolayers, enabling also the less experienced researchers to obtain reproducible results within a short period of time, and which should simplify the comparison of future studies between labs, but also encourage others to consider these systems as alternative cell culture models in their research.
Graphic abstract:
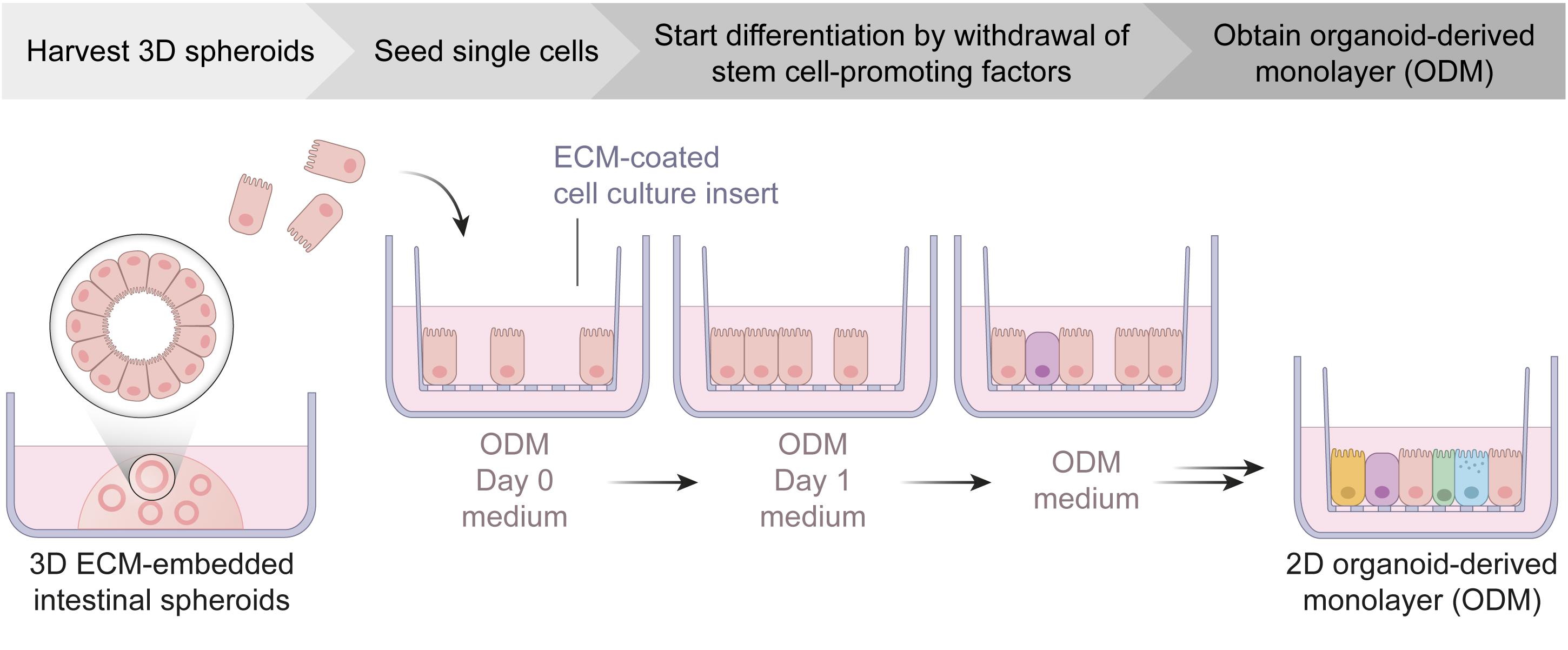
Schematic workflow of organoid-derived monolayer generation from intestinal spheroid cultures. ECM, extracellular matrix; ODM, organoid-derived monolayer.
Background
The small intestinal epithelium is the primary route of infection for many pathogens. In the past, the understanding of host-pathogen interactions has mostly relied on immortalized cell lines or mouse models, which not only lack the complexity of the intestinal epithelium, but also have shortcomings with regard to translatability to the in vivo situation in humans (Klotz et al., 2012; Delgado-Betancourt et al., 2019; Bar-Ephraim et al., 2020; Holthaus et al., 2020). Starting with the development of organoid models by Sato et al. (2009), these concerns have been addressed by many groups, and resulted in the implementation of organoid models for the study of pathogenic bacteria, viruses, and parasites (Bartfeld, 2016; Dutta and Clevers, 2017; Hill and Spence, 2017). Importantly, they are no longer relying solely on the mouse or other animal models, but are moving towards relevant hosts for respective diseases, an important aspect especially in the context of zoonotic pathogens.
There are a multitude of protocols for the establishment of organoid models to study host-pathogen interactions and several labs have developed protocols for the generation of two-dimensional organoid-derived monolayers that provide direct experimental access to the apical and/or basolateral side and allow the study of physiological processes and tissue barrier functions (Moon et al., 2014; VanDussen et al., 2015; Kozuka et al., 2017). However, many protocols focus on only one species, which requires adaptations to suit species-specific needs in case of model switching. Moreover, there is no simple and concise guide to follow, due to the many variations introduced mainly by labs studying a particular host. Here, we provide a protocol using a single common base medium that was tested for four species that play an important role in infectious disease research, namely human, mouse, chicken, and pig.
The starting material for the generation of organoid-derived monolayers are intestinal spheroids, which are stem cell-enriched non-budding three-dimensional structures, obtained by culturing intestinal stem cells in an extracellular matrix hydrogel with Wnt-3a-supplemented media (Miyoshi and Stappenbeck, 2013; VanDussen et al., 2015 and 2019). The term organoid, in a strict sense, refers to more heterogeneous budding structures composed of fewer stem cells and more differentiated epithelial cells, but is also often more broadly used to describe this type of three-dimensional cell culture system.
To achieve intestinal monolayers with a physiological cell composition, differentiation is induced by omitting stem cell-promoting factors from the medium. Previous work by Sato et al. (2009) and Kozuka et al. (2017) provides an excellent overview of the influence of medium conditions on differentiation, and was used as the basis for composing the media in this protocol. We tested the proposed media conditions in our system, and found that the medium we used [withdrawal of Wnt-3a, transforming growth factor beta (TGF-β) receptor signaling inhibitor A83-01, and p38 inhibitor SB202190, but presence of nicotinamide] provided an electrophysiologically thight epithelium, allowed enterocyte-guided differentiation, and enabled prolonged culture of human, mouse, chicken, and pig ODMs for at least six days (Holthaus et al., 2020). With our protocol, one is able to seed and maintain confluent organoid-derived monolayers from human, mouse, chicken, and pig that resemble physiological properties and cell composition, providing an accessible platform for further optimizations and adaptations required for different research approaches. Examples for the application of this protocol can be found in Kraft et al. (2020) and Holthaus et al. (2020).
Materials and Reagents
Materials for organoid culture
12/24-well tissue culture plates (F-base; TPP, catalog numbers: 920 12/24)
15/50 mL conical centrifuge tubes (TPP, catalog number: 910 15/50)
1.5 mL microcentrifuge tubes (BrandTech, catalog number: 780500)
10/200/1,000 μL ART barrier pipette tips (Thermo Scientific, catalog numbers: 10098960/10029040/10313272)
20/100 μL PP filter tips (Nerbe Plus, catalog numbers: 07-662-8300/07-642-8300)
PCF Transwell inserts (0.6 cm2, 0.4 μm pores; Merck, Millicell, catalog number: PIHP01250)
Vacuum filtration system "rapid"-Filtermax (0.22 μm; TPP, catalog number: 99505)
Sartorius MinisartR sterile filters (0.22 μm; Thermo Scientific, catalog number: 10730792)
Disposable hemocytometer (Kisker Biotech, catalog number: M-NZ)
Duran glass beakers (25/600-mL; Schott, catalog numbers: 2110614, 2110648)
Duran glass bottles (500/1,000-mL; Schott, catalog numbers: 4459/5455)
Bemis Parafilm "M" (Thermo Scientific, catalog number: 11762644)
B Braun solo cone Luer syringes (5/10/20-mL; Braun, catalog number: 12752637)
Sterican single-use 21 G syringe needles (Blunt, 25 mm, 0.4 mm; Carl Roth, Braun, catalog number: X134.1)
Single-use 18 G syringe needles (Blunt, 40 mm; VWR, BD Medical, catalog number: BDAM303129)
Note: Examples for providers/manufacturers are given; similar material from others might work as well.
Reagents for organoid culture
Advanced DMEM/F-12 (Thermo Scientific, Gibco, catalog number: 12634028; store at 4°C)
Recombinant Human EGF (Peprotech, catalog number: AF-100-15; store lyophilized at -20°C and aliquots at -80°C)
Recombinant Murine EGF (Peprotech, catalog number: 315-09; store lyophilized at -20°C and aliquots at -80°C)
N-Acetyl-L-cysteine (Sigma-Aldrich, catalog number: A9165; store powder at 4°C and aliquots at -80°C)
Nicotinamide (Sigma-Aldrich, catalog number: N0636; store powder at room temperature and aliquots at -80°C)
A83-01 (Sigma-Aldrich, catalog number: SML0788; store lyophilized at -20°C and aliquots at -80°C)
SB 202190 (Cayman chemicals, catalog number: 10010399-10; store lyophilized at -20°C and aliquots at -80°C)
B-27 Supplement (Thermo Scientific, Gibco, catalog number: 17504044; store at -80°C)
N-2 Supplement (Thermo Scientific, Gibco, catalog number: 17502048; store at -80°C)
HEPES (1 M; Thermo Scientific, Gibco, catalog number: 15630056; store at 4°C)
GlutaMAX Supplement (100×; Thermo Scientific, Gibco, catalog number: 35050038; store at 4°C)
Penicillin/Streptomycin (100×; penicillin [10,000 U/mL] and streptomycin [10,000 µg/mL]; Capricorn scientific, catalog number: PS-B; store at -20°C)
Y-27632 (hydrochloride; Cayman chemicals, catalog number: 10005583; store lyophilized at -20°C and aliquots at -80°C)
TrypLE Express Enzyme (1×; Thermo Scientific, Gibco, catalog number: 12605010; store at 4°C)
ECM Matrigel (Corning, catalog number: 734-0269; store aliquots at -20°C) or Cultrex Reduced Growth Factor Basement Membrane Extract Type 2 (Bio-Techne, R&D Systems, catalog number: 3533-010-02; store aliquots at -80°C)
Note: A protein content of ≥ 8.5 mg/mL is desirable.
Bovine Serum Albumin (BSA, fatty acid free; Sigma-Aldrich, catalog number: A7030-100G; store at 4°C)
Dimethyl sulfoxide (DMSO; Sigma-Aldrich, catalog number: D2650-100ML; store at room temperature)
80% ethanol or other equivalent disinfectant, store at room temperature
MilliQ or other equivalent sterile water, store at room temperature
Note: Found to work as described; similar reagents from other providers/manufacturers might work as well.
Reagents for production of conditioned medium
DMEM High Glucose (4.5 g/L), with Stable Glutamine (Capricorn Scientific, catalog number: DMEM-HPSTA; store at 4°C)
DMEM, high glucose with GlutaMAX Supplement and pyruvate (Thermo Scientific, Gibco, catalog number: 31966047; store at 4°C)
Fetal Bovine Serum (FBS; Capricorn scientific, catalog number: FBS-LE-12A; store aliquots at -20°C)
Note: Test new FBS batches for a minimum of three weeks before use in routine culture. Heat inactivate for 30 min at 56°C and store 50 mL aliquots at -20°C.
Trypsin-EDTA (Capricorn scientific, catalog number: TRY-1B10; store aliquots at -20°C)
Phosphate-buffered saline (PBS; made in-house; store at room temperature or at 4°C)
Penicillin/Streptomycin (100×, penicillin [10,000 U/mL] and streptomycin [10,000 µg/mL]; Capricorn scientific, catalog number: PS-B; store aliquots at -20°C)
Hygromycin B (50 mg/mL; Carl Roth, catalog number: CP12.2; store at 4°C)
Geneticin (G418; 50 mg/mL; Carl Roth, catalog number: CP11.2; store at 4°C)
Zeocin (Invivogen, catalog number: ant-zn-1; store aliquots at -20°C)
Luciferase Assay System (Promega, catalog number: E1500; store aliquots at -20°C)
Note: Found to work as described; similar reagents from other providers/manufacturers might work as well.
Cell lines for conditioned media and its derived media
L-WRN (ATCC CRL-3276) (VanDussen et al., 2019)
HEK 293T HA-noggin-Fc (Heijmans et al., 2013; Bartfeld et al., 2015)
Kindly provided by Hans Clevers, Hubrecht Institute, Utrecht.
HEK 293T R-Spondin1-Fc (Kim et al., 2005)
Kindly provided by Calvin Kuo, Stanford University, USA (subject to MTA).
HEK 293 SuperTopFlash (ATCC CRL-3249)
Luciferase reporter cell line for activity testing of L-WRN and R-Spondin 1 conditioned media.
WERN 3D base proliferation medium for spheroid culture (see Recipes)
Organoid freezing medium (see Recipes)
Dissociation reagent (see Recipes)
ODM Day 0 medium (see Recipes)
ODM Day 1 medium (see Recipes)
ODM medium, day 2 onwards (see Recipes)
Phosphate buffered saline (see Recipes)
L-WRN growth medium (see Recipes)
L-WRN conditioning medium (see Recipes)
R-Spondin 1 and mNoggin growth medium (see Recipes)
R-Spondin 1 and mNoggin conditioning medium (see Recipes)
L-WRN, R-Spondin 1, and mNoggin freezing medium (see Recipes)
Equipment
-20°C freezer
Vacuum Aspirator system, Integra Biosciences Vacusafe Comfort Aspiration system (Fisher Scientific, Integra, catalog number: 10590273)
Adjustable pipettes, Eppendorf Research plus 10/20/200/1,000 pipettes (Eppendorf, catalog numbers: 3123000020, 3123000039, 3123000055, 3123000063)
CO2 cell culture incubator (Humidified atmosphere, 5% CO2, 37°C), CB 170 CO2 incubator (Binder, model number: CB170-230V-O)
Safety cabinet for BSL-2 work, Safe 2020 Class II Biological Safety Cabinet (Thermo Scientific, catalog number: 51026640)
Cooled centrifuge for 15/50 mL conical tubes, Multifuge X1R Multi-Application Centrifuge (Thermo Scientific, catalog number: 15364306)
Cooling containers, Eppendorf IsoRack with IsoPack 0°C (Eppendorf, catalog number: 3880001174)
CoolCell LX Cell Freezing Vial Containers (Fisher Scientific, Corning, catalog number: 15572771)
37°C water bath, WNB 14 (Carl Roth, Memmert, model: WNB 14)
Luminometer, TriStar LB 941 multimode microplate reader (Berthold, model: LB 941)
Chopstick electrode for measurement of transepithelial electrical resistance, Volt-Ohm meter ERS-2 with electrode STX01 (Merck, Millicell, catalog number: MERS00002)
37°C heating block for measurement of transepithelial electrical resistance, digital dry bath incubator (MRC Laboratory Instruments, model: DBD-002N)
Note: Examples for providers/manufacturers are given; similar material from others will probably work as well.
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Warschkau, D., Delgado-Betancourt, E., Holthaus, D., Müller, A., Kliem, G., Krug, S. M., Schulzke, J. D., Aebischer, T., Klotz, C. and Seeber, F. (2022). From 3D to 2D: Harmonization of Protocols for Two-dimensional Cultures on Cell Culture Inserts of Intestinal Organoids from Various Species . Bio-protocol 12(2): e4295. DOI: 10.21769/BioProtoc.4295.
分类
干细胞 > 成体干细胞 > 肠道干细胞
微生物学 > 微生物-宿主相互作用 > 体外实验模型
细胞生物学 > 细胞分离和培养 > 3D细胞培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link