- EN - English
- CN - 中文
Label-free Quantification of Direct Protein-protein Interactions with Backscattering Interferometry
使用反向散射干涉测量法对直接蛋白质-蛋白质相互作用进行无标记量化
发布: 2021年12月20日第11卷第24期 DOI: 10.21769/BioProtoc.4256 浏览次数: 3352
评审: Manjula MummadisettiPooja VermaBhanu Jagilinki

相关实验方案
Cell-Sonar:通过特定蛋白标志物表达变化追踪目标蛋白的简便低成本方法
Sabrina Brockmöller [...] Simone Rothmiller
2025年02月05日 1654 阅读
Abstract
The functional performance of a cell depends on how macromolecules, in particular proteins, come together in a precise orientation, how they assemble into protein complexes and interact with each other. In order to study protein-protein interactions at a molecular level, a variety of methods to investigate these binding processes yield affinity constants and/or the identification of binding regions. There are several well-established biophysical techniques for biomolecular interaction studies, such as fluorescence spectroscopy and surface plasmon resonance. Although these techniques have been proven to be efficient, they either need labeling or immobilization of one interaction partner. Backscattering interferometry (BSI) is a label-free detection method, which allows label- and immobilization-free interaction analysis under physiologically relevant conditions with high sensitivity and in small volumes. We used BSI to measure the interaction of the neuronal calcium sensor recoverin with its target G protein-coupled receptor kinase 1 (GRK1) as a model system. Increasing concentrations of purified recoverin were mixed with a specific concentration of a GRK1 fusion protein. In this protocol, we provide a full description of the instrumental setup, data acquisition, and evaluation. Equilibrium dissociation constants of recoverin-GRK1 interaction determined by the BSI instrumental setup are in full agreement with affinity constants obtained by different methods as described in the literature.
Background
Molecular binding interactions are based on driving forces set by hydrogen bonding, hydrophobic and electrostatic interactions, and van der Waals and London dispersion forces. These interactions are fundamental in cellular processes and have led to several significant discoveries in the fields of biochemistry, proteomics, chemistry, and biophysics (Archakov et al., 2003). The struggle to find label-free approaches for interaction studies has remained a major goal in the last three decades. Techniques such as NMR studies (Ames et al., 1994), mass spectrometry (Anderson et al., 2014), surface plasmon resonance (Dell’Orco and Koch, 2016), biolayer interferometry (Kumar et al., 2016), and backscattering interferometry (Bornhop et al., 2007 and 2016; Sulmann et al., 2017) are currently among the most applied methods. Backscattering interferometry (BSI) is a relatively new method and is known for its high sensitivity, immobilization-free, and label-free quantification to study protein-protein or protein ligand interactions. BSI has a refractive index (RI) detector, which measures protein-protein interactions and can also be used to study protein-ligand interactions (Bornhop et al., 2016). More recently, BSI has been successfully used in drug discovery studies (Mizuno et al., 2019), as well as protein-small molecule and protein-ion interactions (Kammer et al., 2018). The current detection limit of the BSI signal is ΔRI = 10–6. ΔRI is defined as the relative difference in refractive index between two samples having nearly identical RI values. For example, comparing RI values of 1.391312 with 1.391318 yields a ΔRI = 6 × 10-6 (Bornhop et al., 2007).
Recoverin is a neuronal calcium sensor that interacts with G protein-coupled receptor kinase 1 (GRK1, rhodopsin kinase) in a calcium dependent manner. The interplay of recoverin and GRK1 is important for primary visual processes, as it controls the phosphorylation of rhodopsin (Gorodovikova et al., 1994; Klenchin et al., 1995). Phosphorylation of rhodopsin and several feedback loops are important control steps in photoresponse recovery (Koch and Dell’Orco, 2015). Previous work revealed that the N-terminal amphipathic α-helix of GRK1 comprising the first 25 amino acids is essential for the interaction, as it comes into contact with a hydrophobic pocket in recoverin (Figure 1) (Ames et al., 2006; Higgins et al., 2006). Recoverin-GRK1 complex formation is under the control of calcium. At high saturating calcium concentrations, recoverin exposes its covalently attached myristoyl-group, opening the hydrophobic pocket for the N-terminus of GRK1. In this state, GRK1 is inhibited and unable to phosphorylate rhodopsin. This process is reversed when the calcium concentration drops (Komolov et al., 2009; Zernii et al., 2011). More recently,Abbas et al. (2019) reported that the interaction between recoverin and GRK1 involves a network of switching electrostatic interactions in recoverin by using BSI. Thus, Figure 1 represents the optimal structural configuration of the recoverin-GRK1 complex. By employing a combinatorial approach using BSI and various other techniques, we have further investigated the complex formation using wild-type and mutant forms of recoverin. Results from surface plasmon resonance, isothermal titration calorimetry, tryptophan fluorescence spectroscopy, CD spectroscopy, and molecular dynamics simulations were all in agreement with our BSI studies. The calcium-dependent interaction of recoverin with its target region in GRK1 (peptide encompassing 25 amino acids of the N-terminus) is well suited as a benchmark example for establishing BSI in any laboratory.
In this protocol, we employ BSI to study biomolecular interactions between purified recoverin and the N-terminus of GRK1 containing the first 25 amino acids N-terminally tagged with glutathione-S-transferase (GST-NRK25). We provide a step-by-step protocol including the instrument setup, the detection of signals, designing a binding experiment, and data analysis.
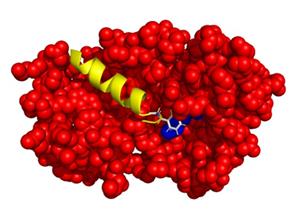
Figure 1. Possible interaction between Recoverin-GRK1. Recoverin is represented by red spheres. The blue spheres represent the C-terminal region of recoverin that interacts with GRK1. GRK1 consists of 558 amino acids and the interaction domain (M1-S25), shown as a yellow coloured helix, is present in the hydrophobic pocket of the protein recoverin.
Materials and Reagents
Eppendorf tubes (SARSTEDT)
Pipette tips 10 µl (Sartorius)
Pipette tips 200 µl (Sartorius)
Pipette tips 1,000 µl (Sartorius)
Pipette set (SARSTEDT, Nümbrecht)
Falcon tubes 50 ml (DARSTEDT AG and CO.KG, catalog number: 9042211)
Photometer cuvettes (Ratiolabs, catalog number: 2712120)
Glass beaker (Schott Duran)
Disposable vinyl gloves (Semper guard)
Lens cleaning wipe papers (Thorlabs, Inc.)
Ethanol (Sigma-Aldrich, catalog number: STBJ2652)
HCl (VWR chemicals 25% solution, catalog number: 20257-296)
Acetone 99.5% (Sigma-Aldrich, catalog number: STBJ1946)
KOH (Roth, catalog number: 6751.1)
HEPES (Roth, catalog number: 9105.2)
Magnesium chloride (MgCl2) (VWR Chemicals, catalog number: 25108.295)
Calcium chloride (CaCl2) (Roth, catalog number: 5239.1)
Potassium chloride (KCl) (Apllicem, catalog number: 7447.40.7)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: STB117768)
Bradford reagent (Bio-Rad, catalog number: 500-00069)
BSI buffer (see Recipes)
Equipment
Caramba Druckluftspray for cleaning electronic devices with air pressure (Böttcher AG, Germany)
Head light (Energizer)
Sample labeling marker (STAEDTLER permanent Marker pen)
Lyophylizer (Alpha 1-2 LD plus, Martin Christ Gefriertrocknungsanlagen GmbH, catalog number: 101521)
Vortex (Vortex Genie 2) (Sigma-Aldrich, catalog number: Z258423-1EA)
Sonicator (Bandelin Sonorex)
-20°C freezer (Liebherr)
Photometer (Food ALYT OMNILAB, catalog number: 122197)
Toolbox (Newport)
Microfluidic chip (Micronit-Vanderbilt)
Backscattering Intrument, built according to Bornhop et al. (2007 and 2016) and Kammer et al. (2018) .
Software
Standard FFT program 9.0 Alphas LV82 simple with saving frames
BSI SCSR analysis program-2012 (Bornhop lab at Vanderbilt University, USA)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Abbas, S. and Koch, K. W. (2021). Label-free Quantification of Direct Protein-protein Interactions with Backscattering Interferometry. Bio-protocol 11(24): e4256. DOI: 10.21769/BioProtoc.4256.
分类
生物物理学 > 散射光谱
神经科学 > 细胞机理 > 胞内信号传导
生物化学 > 蛋白质 > 相互作用 > 蛋白质-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link