- EN - English
- CN - 中文
Production, Titration, Neutralisation, Storage and Lyophilisation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Lentiviral Pseudotypes
严重急性呼吸系统综合症冠状病毒 2 (SARS-CoV-2) 慢病毒假型的生产、滴定、中和、储存和冻干
发布: 2021年11月05日第11卷第21期 DOI: 10.21769/BioProtoc.4236 浏览次数: 6130
评审: Khyati Hitesh ShahMeenal SinhaAnonymous reviewer(s)
Abstract
This protocol details a rapid and reliable method for the production and titration of high-titre viral pseudotype particles with the SARS-CoV-2 spike protein (and D614G or other variants of concern, VOC) on a lentiviral vector core, and use for neutralisation assays in target cells expressing angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2). It additionally provides detailed instructions on substituting in new spike variants via gene cloning, lyophilisation and storage/shipping considerations for wide deployment potential. Results obtained with this protocol show that SARS-CoV-2 pseudotypes can be produced at equivalent titres to SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) pseudotypes, neutralised by human convalescent plasma and monoclonal antibodies, and stored at a range of laboratory temperatures and lyophilised for distribution and subsequent application.
Keywords: SARS-CoV-2 coronavirus (SARS-CoV-2冠状病毒)Background
SARS-CoV-2 is the causative agent of COVID-19 disease currently manifesting as a global pandemic (Zhu et al., 2020). Due to the highly infectious nature of SARS-CoV-2, the wild-type virus has been classified as a BSL-3 pathogen, heavily restricting its use in many laboratories. To circumvent this biohazard restriction, pseudotype viruses (PVs) can be generated by utilising a surrogate viral core to generate virions displaying the SARS-CoV-2 spike protein (Nie et al., 2020; Crawford et al., 2020). Due to the single-round infection and replication deficient properties of PVs, they can be employed in BSL-2 laboratories. The use of PVs as a platform to investigate serosurveillance, antigenic properties and viral entry mechanisms of emerging viruses has been extensively reviewed (Bentley et al., 2015; Li et al., 2018; Cantoni et al., 2021; Focosi et al., 2021), with many studies demonstrating a high degree of correlation between wild-type virus neutralisation assays and PV neutralisation assays (Hyseni et al., 2020; Schmidt et al., 2020; Sholukh et al., 2020; Xiong et al., 2020). Furthermore, PVs can be used as a diagnostic control for new platforms to detect SARS-CoV-2 infection in patients (Sholukh et al., 2020).
Since the start of the pandemic, many protocols have been established for generating SARS-CoV-2 pseudotypes using a range of viral cores, all of which have shown that long term storage of PVs is ideal at -80°C (Crawford et al., 2020; Nie et al., 2020). The issue that this presents is that sharing PVs with research or diagnostic laboratories that do not have expertise in PV generation and application is that it would incur high shipping costs, as the particles need to be shipped on dry ice to remain stable. Additionally, many laboratories within low-income and middle-income countries (LMICs) have no routine access to -80 °C storage. In this protocol, we present our method of generating (Figure 1) and using lentiviral based PVs (highly adaptable and biosafe) expressing SARS-CoV-2 spike, with the additional step of lyophilisation of SARS-CoV-2 PVs using sucrose as a cryopreservant and a freeze drier. Our previous experience has shown that lyophilisation of Influenza, Rabies, and Marburg PVs does not affect PV performance (Mather et al., 2014). We show that SARS-CoV-2 PV can be lyophilised in the same manner and can thus be transported at room temperature over extended time periods (at least a week) and reconstituted into solution without any loss of PV performance (for similar PV, reconstitute within a year if kept at 4°C). This will greatly expand the adoption of pseudotype SARS-CoV-2 neutralisation assays globally and facilitate diagnostic platforms that use SARS-CoV-2 PVs as control samples, like MALDI-ToF (Iles et al., 2020), from both a financial and practical point of view.
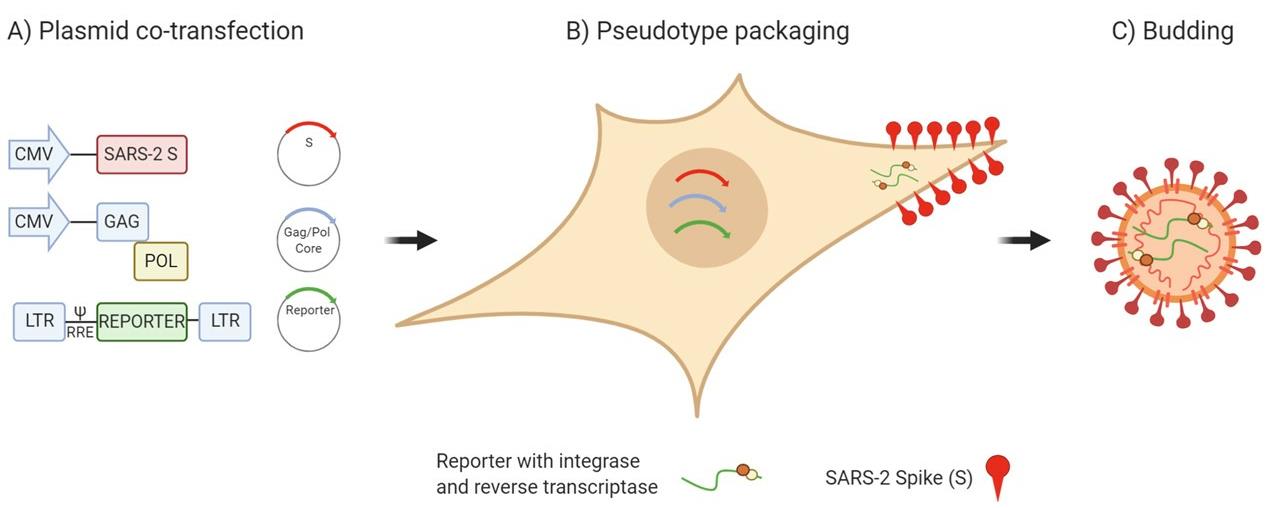
Figure 1. Schematic representation of the production of SARS-CoV-2 pseudotype viruses. HEK293T/17 cells are transfected with three plasmids (Lentiviral vector incorporating luciferase reporter, packaging construct and SARS-CoV-2 spike expression plasmid) for the production of SARS-CoV-2 Spike bearing lentiviral pseudotypes.
Materials and Reagents
MultiGuard Barrier pipette tips 1-20 and 1-200 μl (Sorenson BioScience, catalog number: 30550T)
NuncTM Cell Culture Treated Multidishes (6-well) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 140675)
NuncTM Cell Culture Dish Delta Surface Treated (10 cm sterile dishes) (Thermo Fisher Scientific, Thermo ScientificTM, catalogue number: 150350)
Reaction tube, 1.5 ml with attached cap, graduation and writing area (Greiner Bio-One, catalog number: 616201)
FisherbrandTM Sterile Syringes for Single Use 3 ml, (Fisher Scientific, Thermo ScientificTM, catalog number: 14955457)
0.45 μm syringe filter, cellulose acetate (STARLAB, catalog number: E4780-1453)
Pipette basins (50 ml), Generic
96-well white plate (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 136101)
Microtube (1.5 ml, 0.5 ml), generic
Thin-walled PCR microtubes (0.2 ml), generic
HEK 293T/17 cells (ATCC, catalog number: CRL-11268)
Subcloning efficiency E. coli DH5α cells (Invitrogen, catalog number: 18265017)
Plasmids
Spike plasmid: pCAGGS-SARS-CoV spike (CFAR, catalog number: 100976)
Lentiviral vector expressing firefly luciferase: pCSFLW (or pCSGW for GFP PV) (Carnell et al., 2015)
Second-generation lentiviral packaging construct plasmid: p8.91 (expresses gag, pol and rev) (Carnell et al., 2015)
Host cell entry receptor ACE2 expression plasmid: pCDNA3.1+-ACE2 (Simmons et al., 2004)
Coronavirus Spike (S) protein priming TMPRSS2 expression plasmid: pCAGGS-TMPRSS2 (Bertram et al., 2010)
Note: Information on the plasmids above can be found in Temperton et al. (2005) and Carnell et al. (2015). Plasmids available from VPU.
Dulbecco’s modified Eagle medium (DMEM) with 4.5 g/L Glucose (Pan-Biotech, catalog number: P04-04510) supplemented with 10% foetal bovine serum (FBS) (Pan-Biotech, catalog number: P40-37500) and 1% penicillin/streptomycin (P/S) (Pan Biotech, catalog number: P06-07100)
Gibco Reduced Serum media Opti-MEM® (Thermo Fisher Scientific, catalog number: 51985034)
FuGENE® HD Transfection Reagent, 1 ml (Promega, catalog number: E2311)
Dulbecco's phosphate-buffered saline (DPBS) without calcium and magnesium (Pan-Biotech, catalog number: P04-36500)
Trypsin-EDTA (0.05%), phenol red (Pan-Biotech, catalog number: P10-040100)
Positive control antibody (Research Reagent for Anti-SARS-CoV-2 Ab) that can neutralise the SARS-CoV-2 PV (NIBSC, code: 20/130, available internationally)
COVID-19 human convalescent plasma panel (NIBSC, catalog number: 20/118)
Monoclonal antibodies that can neutralise the SARS-CoV-2 PV (Native Antigen, catalog numbers: MAB12443 and MAB12444)
Bright GloTM luciferase assay system (Promega, catalog number: E2650)
Low surface tension polypropylene 1.5 ml microtubes (Simport, catalog number: T330-7LST)
Sucrose (Sigma-Aldrich, catalog number: S0389)
Dulbecco’s Phosphate Buffered Saline (DPBS; Pan Biotech, catalog number: P04-361000)
Spike plasmid: pCAGGS-SARS-CoV spike (CFAR, catalog number: 100976)
Eukaryotic expression vector recipient plasmids: pcDNA3.1, pI.18, pCAGGS (Carnell et al., 2015)
Anza enzyme kit (Thermo Fisher Scientific, catalog number: IVGN3006)
Nuclease-free water (Thermo Fisher Scientific, catalog number: R0582)
QIAquick PCR Purification kit (Qiagen, catalog number: 28104)
QIAquick Gel Extraction kit (Qiagen, catalog number: 28704)
Tris Acetate EDTA (TAE) buffer (50× concentrate; Thermo Fisher Scientific, catalog number: B49)
Ultrapure Agarose (Thermo Fisher Scientific, catalog number: 16500100)
SYBR Safe DNA gel stain (Invitrogen, catalog number: S33102)
GeneRuler 1 kb DNA Ladder (Thermo Fisher Scientific, catalog number: SM0313)
Luria Broth (LB) and LB agar powder (Sigma-Aldrich, catalog numbers: L3022 and L2897)
DreamTaq Green PCR Master Mix (Thermo Fisher Scientific, catalog number: K1081)
Monarch plasmid miniprep kit (New England Biolabs, catalog number: T1010S) or alternative
Equipment
Class II biosafety cabinet (Thermo Fisher Scientific, Thermo ScientificTM, model: MSC-AdvantageTM)
Water bath or incubator, generic
Pipettes (Gilson, models: PIPETMAN® Classic, P2, P20, P200 and P1000 or equivalent)
Multichannel pipette (Glison, model: PIPETMAN L Multichannel P12 20-200 μl or equivalent)
FisherbrandTM Sterile Polystyrene Disposable Serological Pipets 5 ml and 10 ml in 1/10 ml, Sterile, Plugged, Individually Wrapped (Fisher Scientific, Thermo ScientificTM, catalog numbers: 1367610H and 1367610J)
Portable Pipet-Aid® XP Pipette Controller (Drummond Scientific Company, catalog number: 4-000-101 or equivalent)
Vortex Mixer, adjustable speed (SciQuip, model: SP2260-VM)
Galaxy MiniStar Mini Centrifuge (VWR, model: C1413V-230)
Optional: BIO-RAD TC20TM Automated Cell Counter (Bio-Rad Laboratories, catalog number: 1450102EDU) or FastRead 102 disposable 10-chamber counting grid with integral acrylic, optically clear, coverslip (Immune Systems, catalog number: BVS100)
Plate centrifuge (ELMI, model: SkyLine CM-6MT)
GloMax® Navigator Microplate Luminometer (Promega, model: GloMax® Navigator)
FreeZone 2.5 L freeze dryer (LabConCo, catalog number: 7670520)
Sample drying chamber (LabConCo, catalog number: 7318700)
Rotary Vane Vacuum pump 117 (LabConCo, catalog number: 7739402)
Plastic microtube rack (Thermo Fisher Scientific, catalog number: 8850)
Thermo-humidity meter (Thermo Fisher Scientific, catalog number: 11536973)
Water bath or heat block, generic
Microwave, generic
Powerpack, generic
Gel electrophoresis tank, generic
Microcentrifuge (Thermo Fisher Scientific, SorvallTM LegendTM)
UV transilluminator (Uvitech/Sigma, catalog number: Z363677)
Gel imaging system, generic
Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, catalog number: ND-2000)
Dry 37°C incubator, generic
Shaking 37°C incubator, generic
Thermocycler (Techne, model: Prime, catalog number: 5PRIMEG/02)
Software
PC or Mac with Microsoft Excel (Microsoft®)
GraphPad Prism® (GraphPad Software)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Di Genova, C., Sampson, A., Scott, S., Cantoni, D., Mayora-Neto, M., Bentley, E., Mattiuzzo, G., Wright, E., Derveni, M., Auld, B., Ferrara, B. T., Harrison, D., Said, M., Selim, A., Thompson, E., Thompson, C., Carnell, G. and Temperton, N. (2021). Production, Titration, Neutralisation, Storage and Lyophilisation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Lentiviral Pseudotypes. Bio-protocol 11(21): e4236. DOI: 10.21769/BioProtoc.4236.
分类
分子生物学 > DNA > 转染
微生物学 > 微生物生物化学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link