- EN - English
- CN - 中文
Imaging of Lipid Uptake in Arabidopsis Seedlings Utilizing Fluorescent Lipids and Confocal Microscopy
利用荧光脂质和共聚焦显微镜对拟南芥幼苗的脂质摄取进行成像
发布: 2021年11月20日第11卷第22期 DOI: 10.21769/BioProtoc.4228 浏览次数: 4029
评审: Khyati Hitesh ShahAksiniya AsenovaAnonymous reviewer(s)
Abstract
Eukaryotic cells use a diverse set of transporters to control the movement of lipids across their plasma membrane, which drastically affects membrane properties. Various tools and techniques to analyze the activity of these transporters have been developed. Among them, assays based on fluorescent phospholipid probes are particularly suitable, allowing for imaging and quantification of lipid internalization in living cells. Classically, these assays have been applied to yeast and animal cells. Here, we describe the adaptation of this powerful approach to characterize lipid internalization in plant roots and aerial tissues using confocal imaging.
Graphic abstract:
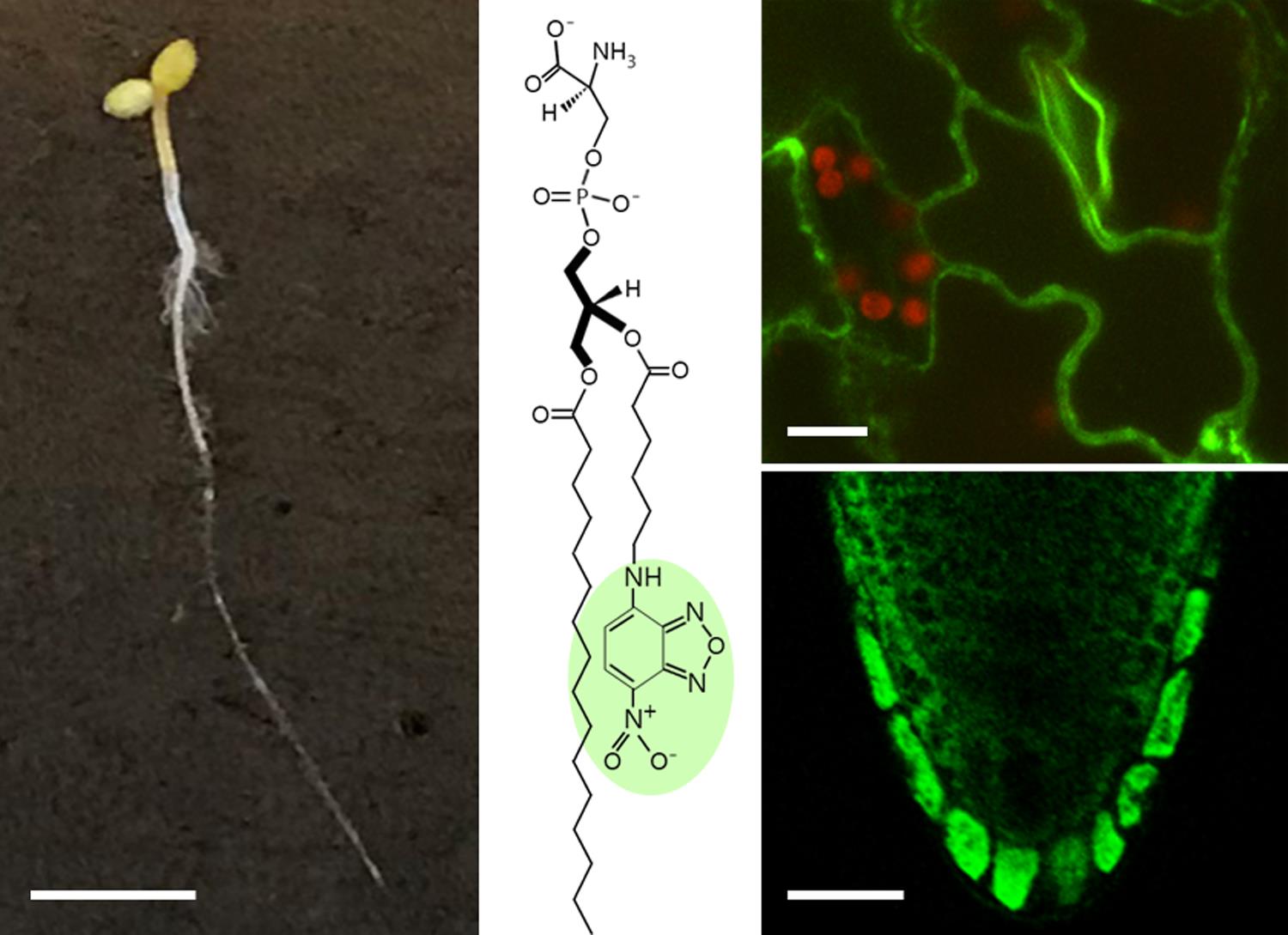
Fluorescent lipid uptake in Arabidopsis seedlings. Scale bars: seedling, 25 mm; leaf, 10 μm; root, 25 μm.
Background
In eukaryotic cells, movement of lipids across biological membranes (known as lipid flip-flop) is regulated by a diverse set of membrane transporters that can be classified into two categories: (i) ATP-independent transporters, also called scramblases, that facilitate a rapid bi-directional movement of lipids without metabolic energy input, and (ii) ATP-driven vectorial transporters that actively translocate lipids from one membrane leaflet to the other, often with high specificity. The latter group comprises ATP-dependent flippases and floppases, which catalyze the inward movement of lipids to the cytoplasmic membrane leaflet, and the outward movement to the extracellular/luminal side, respectively. A subgroup of P-type ATPases, the P4 ATPases, has emerged as a major group of lipid flippases that form heterodimeric complexes with members of the Cdc50 (cell division control 50) protein family (reviewed in Lopez-Marques et al., 2014; Andersen et al., 2016). While initially characterized as aminophospholipid flipases, recent studies of individual family members from fungi, plants, and animals show that P4 ATPases differ in their substrate specificities and mediate transport of a broader range of lipid substrates, including lysophospholipids, synthetic alkylphospholipids, and sugar-modified ceramides (Roland et al., 2019; Shin and Takatsu, 2019).
Quantitative assessment of P4 ATPase lipid transport activity is essential for the determination of substrate specificities and to establish whether and how this activity is regulated in the living cell. As P4-ATPases are trapped in an environment (cellular membranes) formed by their own substrate (lipids), analyzing their activity is not a trivial task, and most assays are based on the use of fluorescent lipid analogs, typically nitrobenzoxadiazol (NBD)-labeled lipids. These analogs have a fluorescent reporter group attached to a short-chain fatty acid (C6) and maintain most of the properties of endogenous phospholipids, except that they are more water-soluble, which facilitates incorporation from the medium into the outer monolayer of the plasma membrane.
Traditionally, lipid uptake assays employing NBD-lipids are carried out after heterologous expression of the plant P4 ATPases in yeast strains devoid of their endogenous lipid transporters (for a review, see Nintemann et al., 2019). However, many plant P4 ATPases express poorly, fail to fold, and/or traffic improperly when produced in heterologous systems. In addition, successful expression does not always result in active lipid translocation, probably due to the absence of plant-specific accessory proteins and/or cofactors required for the functioning of plant lipid transporters (McDowell et al., 2015).
The method presented here utilizes C6-NBD-lipids to study lipid internalization in intact plants, exemplified on Arabidopsis seedlings as a model. Small 5-day old seedlings are grown on plates under sterile conditions and then transferred to liquid growth medium supplemented with C6-NBD-lipids. After incubation for the desired time, the seedlings are washed with medium containing small amounts of a specific detergent to remove excess lipids attached to the cell walls. Finally, plants are visualized using confocal microscopy, and the data quantified using imaging software. This protocol can be used to characterize lipid internalization both in roots and aerial tissues and can be easily adapted to other plant species. For experiments in roots, growth of seedlings on agar plates is preferred to regular cultivation on soil, as removal from the soil causes damage to the root surface.
Materials and Reagents
Materials
Arabidopsis seeds (ecotype Col-0 or as desired)
Pipette tips PIPETMAN DIAMOND D10, D200, D1000 (Gilson, catalog numbers: F161630, F161930, F161670)
2-ml microcentrifuge snap-cap tubes with round bottom (e.g., BRAND, microcentrifuge tube, 2 ml with lid, PP; Merck, catalog number: BR780546-500EA)
Circular holder for 2-ml microcentrifuge tubes (e.g., PrepSafeTM microcentrifuge tube mini floating rack, clear; Merck, catalog number: Z756385)
50-ml glass beaker (e.g., BRAND, catalog number: 91217)
Square Petri dishes 120 × 120 × 17 mm, Greiner Bio-One (Fisher Scientific, catalog number: 07-000-330)
Micropore tape (3M, catalog number: 1530-1)
Aluminum foil (e.g., Sigma, catalog number: Z185140-1EA)
Centrifuge glasses DURAN® with conical bottom, 12 ml (Carl Roth, catalog number: K211.1)
25-μl calibrated glass syringe (model 702 N; Hamilton, catalog number: CAL80400)
1.5 ml screw amber glass vials with 8-mmTeflon-lined screw caps (e.g., VWR, catalog numbers: VWRI548-0019 and 548-0360)
Corning® Pasteur pipettes, non-sterile, 228 mm (Merck, catalog number: CLS7095B9)
PARAFILM® M (Merck, catalog number: P7793)
Clear glass jars with snap-cap, 11 ml, 22 × 45 mm (VWR, catalog number: 548-0625)
For aerial tissues, microscope slides 76 × 26 × 1 mm with cut edges (Histolab, catalog number: 06300)
For root tissues, diagnostic microscope slides 25 × 75 mm, 8 Wells of 6 mm, with black epoxy field around cavities (Histolab, catalog number: 06260)
Cover glass No. 1, 18 × 24 mm and 24 × 24 mm (Histolab, catalog numbers: 06602 and 06608)
Reagents
All reagents can be stored at room temperature, except NBD-phospholipid solutions, which should be kept at -20°C for long-term storage.
Sodium hypochlorite (14% Cl2) in aqueous solution, GPR RECTAPUR® (VWR Chemicals, catalog number: 27900.296)
37% hydrochloric acid solution (Sigma, catalog number: 320331)
Phytoagar (Duchefa Biochemie, catalog number: P1003)
2-(N-morpholino)ethanesulfonic acid (MES) (Merck-Millipore, catalog number: 1061261000; CAS number: 4432-31-9)
Potassium hydroxide (Sigma, catalog number: 484016-1KG; CAS number: 1310-58-3)
Dimethyl Sulfoxide (DMSO), sterile-filtered, BioPerformance Certified (Sigma, catalog number: D2438)
Methanol (Sigma, catalog number: 179337; CAS number: 67-56-1)
Chloroform, ethanol-stabilized and certified for absence of phosgene and HCl (Sigma, catalog number: 650471)
Fluorescent C6-NBD-phospholipids in chloroform
C6-NBD-phosphatidylethanolamine (Avanti Polar Lipids, catalog number: 810153)
C6-NBD-phosphatidylserine (Avanti Polar Lipids, catalog number: 810192)
C6-NBD-phosphatidylcholine (Avanti Polar Lipids, catalog number: 810130)
NBD-lysophosphatidylcholine (Avanti Polar Lipids, catalog number: 810128)
C6-NBD sphingomyelin (Avanti Polar Lipids, catalog number: 810218)
Tergitol solution type NP-40 (Sigma, catalog number: NP40S)
Low-melting-point agarose, analytical grade (Promega Corporation, catalog number: V2111)
Murashige and Skoog (MS) salts with vitamins (Phyto Technology Laboratories, catalog number: M519)
Half strength MS liquid medium (see Recipes)
Half strength MS plates (see Recipes)
Agarose solution (see Recipes)
C6-NBD-lipid stocks (see Recipes)
Equipment
Pipettes PIPETMAN Classic P2, P20, P200, P1000 (Gilson, models: F144801, F123600, F123601, F123602)
Precision tweezers Style #5, fine needle-sharp, anti-magnetic stainless steel (Merck, catalog number: T4537)
Scalpel (e.g., Sigma, catalog numbers: S2646 and S2896)
Glass dessicator (e.g., Boro 3.3 dessicator 20 cm with knob lid; BRAND, catalog number: 65038)
Shallow water bath (e.g., Precision GP 2S, 2L shallow water bath; ThermoFisher, catalog number: TSGP2S)
Incubator or heating block at 60°C (e.g., VWR, catalog number: 75838-270)
Analytical balance (e.g., Sartorius Entris-i II, 220 g/0.1 mg, Buch Holm, catalog number: 4669128)
Autoclave sterilizer (e.g., Presoclave III, 80 liters, Ø: 40 × 62 cm, Buch Holm, catalog number: 5083042)
Freezer (e.g., GRAM Bioline, model: BioCompact 210RF)
Refrigerator (e.g., GRAM Bioline, model: BioCompact 210RR)
Rotary evaporator equipped with vacuum pump or nitrogen gas supply [e.g., Büchi® Rotavapor® RII evaporator with jack and water bath (Sigma, catalog number: Z564036), equipped with a Vacuubrand diaphragm vacuum pump model MD1C (Sigma, catalog number: Z656194)]
Water purification systems (e.g., Milli-Q® Direct water purification system, Merck-Millipore, catalog number: ZR0Q008WW)
Fume hood (e.g., ErlabTM Captair 391 Smart Fume Hood, Fisher Scientific, catalog number: 15514360)
Microwave oven (e.g., H2100 Microwave Oven 220 Volt, Merck, catalog number: A9209)
Laminar flow cabinet (e.g., Fortuna Clean Bench, ScanLaf, Labogene)
Plant growth chamber (e.g., Sanyo Versatile Environmental Test Chamber, model: MLR-351H)
Confocal microscope, e.g., Leica TCS SP5 Confocal Laser Scanning Microscope equipped with an argon laser (Leica Microsystems A/S) and a C-Apochromat 63×/1.2 W autocorr M27 (CG=0.14-0.19 mm) (FWD=0.28 mm at CG=0.17 mm) objective (Carl Zeiss Microscopy, catalog number: 421787-9971-790)
Software
Leica LAS X software, version 3.5.7.23225 (Leica Microsystems A/S)
ImageJ 1.52n (National Institutes of Health, USA, http://imagej.nih.gov/ij) (Schneider et al., 2012)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
López-Marqués, R. L. and Pomorski, T. G. (2021). Imaging of Lipid Uptake in Arabidopsis Seedlings Utilizing Fluorescent Lipids and Confocal Microscopy. Bio-protocol 11(22): e4228. DOI: 10.21769/BioProtoc.4228.
分类
植物科学 > 植物细胞生物学 > 细胞成像
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link