- EN - English
- CN - 中文
Development of a 3D Bioprinted Scaffold with Spatio-temporally Defined Patterns of BMP-2 and VEGF for the Regeneration of Large Bone Defects
开发具有 BMP-2 和 VEGF 时空定义模式的 3D 生物打印支架,用于大骨缺损的再生
发布: 2021年11月05日第11卷第21期 DOI: 10.21769/BioProtoc.4219 浏览次数: 3386
评审: Giusy TornilloJaira Ferreira de VasconcellosAnonymous reviewer(s)
Abstract
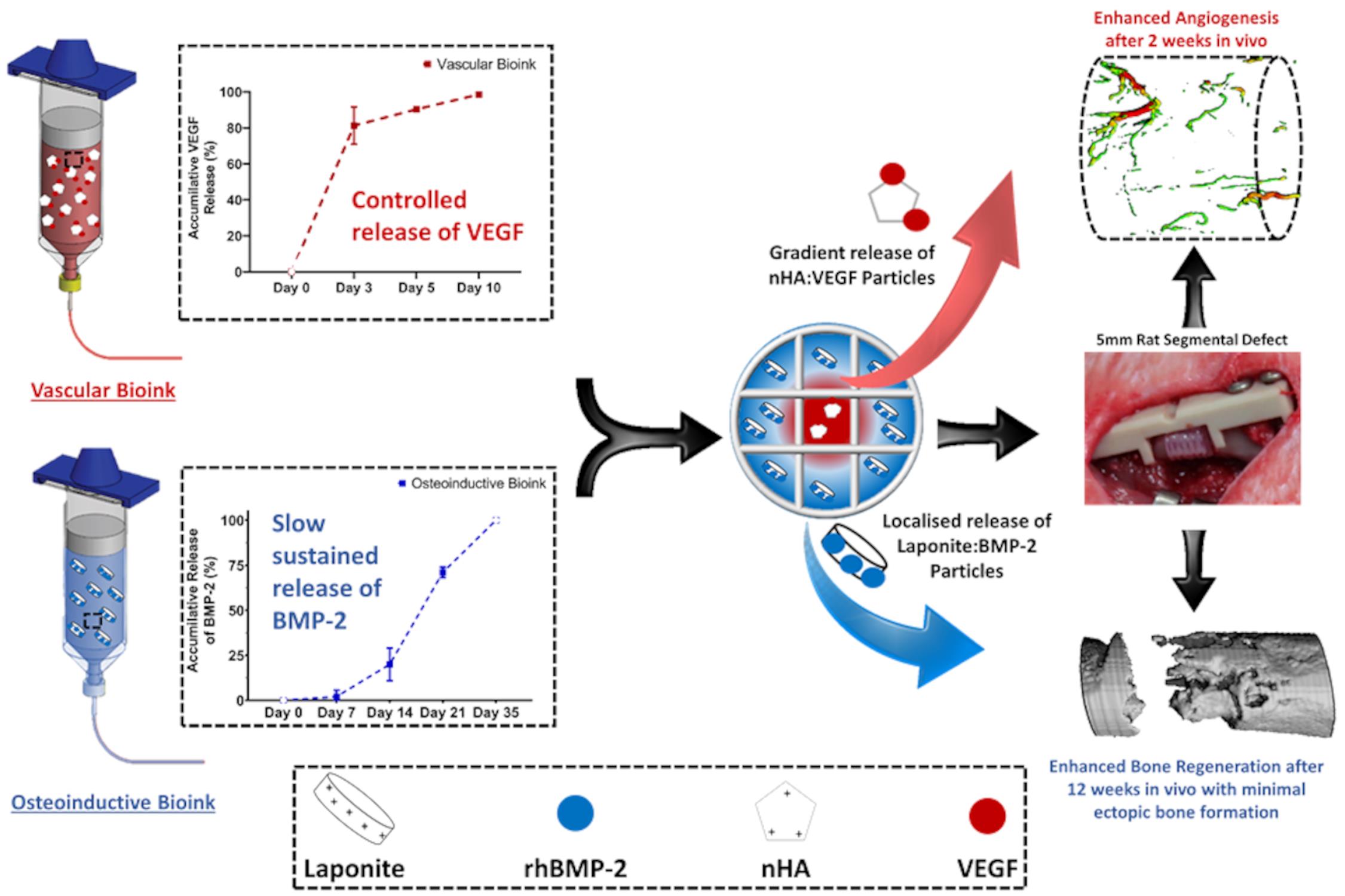
The local delivery of growth factors such as BMP-2 is a well-established strategy for the repair of bone defects. The limitations of such approaches clinically are well documented and can be linked to the need for supraphysiological doses and poor spatio-temporal control of growth factor release in vivo. Using bioprinting techniques, it is possible to generate implants that can deliver cytokines or growth factors with distinct spatiotemporal release profiles and patterns to enhance bone regeneration. Specifically, for bone healing, several growth factors, including vascular endothelial growth factor (VEGF) and bone morphogenic proteins (BMPs), have been shown to be expressed at different phases of the process. This protocol aims to outline how to use bioprinting strategies to deliver growth factors, both alone or in combination, to the site of injury at physiologically relevant dosages such that repair is induced without adverse effects. Here we describe: the printing parameters to generate the polymer mechanical backbone; instructions to generate the different bioinks and allow for the temporal control of both growth factors; and the printing process to develop implants with spatially defined patterns of growth factors for bone regeneration. The novelty of this protocol is the use of multiple-tool fabrication techniques to develop an implant with spatio-temporal control of growth factor delivery for bone regeneration. While the overall aim of this protocol was to develop an implant for bone regeneration, the technique can be modified and used for a variety of regenerative purposes.
Graphic abstract:
3D Bioprinting Spatio-Temporally Defined Patterns of Growth Factors to Tightly Control Bone Tissue Regeneration.
Background
The limitations of local delivery of growth factors clinically are well documented and can be linked to the need for supraphysiological doses. Multiple-tool biofabrication techniques overcome this limitation by making it is possible to generate ‘print-and-implant’ devices to deliver growth factors with distinct spatiotemporal release profiles to enhance tissue regeneration. Specifically, for bone healing, several growth factors have been shown to be expressed at different phases of healing, including vascular endothelial growth factor (VEGF) and bone morphogenetic proteins (BMPs). This protocol describes in detail how we developed a bioinks that interact with the growth factors to make it possible to tightly control the location and timing of their release in vivo, thereby negating the need for supraphysiological doses.
Materials and Reagents
Luer lock syringe cap seal (Adhesive Dispensing, catalog number: 7015LLPK)
10cc clear syringe barrel (Adhesive Dispensing, catalog number: 7100LL1NPK)
10cc syringe piston (Adhesive Dispensing, catalog number: 7100009WPK)
27 G needle, 0.25'' long (Adhesive Dispensing, catalog number: TE727025PK)
5 ml syringe (VWR, catalog number: 613-2043)
RGD γ-irradiated alginate (Manufactured in house by collaborators in Alsberg’s Lab)
Commercially available NOVATACHTM peptide-coupled alginate could also be used, but the crosslinking ratios would need to be modified depending on the MW of the alginate used.
Sodium Triphosphate (Sigma-Aldrich, catalog number: 72061)
Darvan 821AVR (Vanderbilt Minerals, DARVAN® 821-A)
Sodium hydroxide (Sigma-Aldrich, catalog number: S8045)
Vascular Endothelial Growth Factor (Peprotech, catalog number: 100-20)
Bone Morphogenic Protein-2 (Peprotech, catalog number: 120-02)
Methylcellulose (Sigma-Aldrich, catalog number: M7027)
Dulbecco’s modified Eagle medium (GlutaMAXTM; ThermoFisher, catalog number: 61965026)
Fetal bovine serum (FBS; Thermo Scientific, catalog number: 10270-106)
Penicillin streptomycin (Sigma-Aldrich, catalog number: 15070063)
Laponite (Laponite XLG, BYK Additives & Instruments)
Polycaprolactone (PCL; CAPA 6500D, Perstorp, Mn = 50 kDa)
Calcium Chloride (Sigma-Aldrich, catalog number: C5670)
Calcium sulfate (Sigma-Aldrich, catalog number: 255696)
3.5% RGD γ-irradiated alginate solution (see Recipes)
100 mM calcium chloride solution (see Recipes)
60 mM calcium sulphate solution (see Recipes)
Sodium triphosphate solution (see Recipes)
Equipment
Sterile stirrer bar and stirrer plate
3D bioplotter (RegenHU, 3D Discovery, Generation 1)
Software
BioCAD (RegenHU, 3D Discovery, Generation 1)
GraphPad (GraphPad Software, La Jolla, CA, USA; www.graphpad.com)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Freeman, F. E., Pitacco, P., van Dommelen, L. H. A., Nulty, J., Browe, D. C., Shin, J. Y., Alsberg, E. and Kelly, D. J. (2021). Development of a 3D Bioprinted Scaffold with Spatio-temporally Defined Patterns of BMP-2 and VEGF for the Regeneration of Large Bone Defects. Bio-protocol 11(21): e4219. DOI: 10.21769/BioProtoc.4219.
- Freeman, F. E., Pitacco, P., van Dommelen, L. H. A., Nulty, J., Browe, D. C., Shin, J. Y., Alsberg, E. and Kelly, D. J. (2020). 3D bioprinting spatiotemporally defined patterns of growth factors to tightly control tissue regeneration. Sci Adv 6(33): eabb5093.
分类
生物工程 > 生物打印
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











