- EN - English
- CN - 中文
An Assay to Determine NAD(P)H: Quinone Oxidoreductase Activity in Cell Extracts from Candida glabrata
测定 NAD(P)H 的分析:光滑念珠菌细胞提取物中的醌氧化还原酶活性
(*contributed equally to this work) 发布: 2021年11月05日第11卷第21期 DOI: 10.21769/BioProtoc.4210 浏览次数: 3051
评审: Alexandros AlexandratosSascha BrunkeYingnan Hou
Abstract
Flavodoxin-like proteins (Fld-LPs) are an important constituent of the oxidative stress defense system in several organisms and highly conserved from bacteria to humans. These proteins possess NAD(P)H:quinone oxidoreductase activity and convert quinones to hydroquinones through two-electron reduction, using NAD(P)H and quinone as electron donor and acceptor, respectively. Purified yeast and bacterial Fld-LPs exhibit NAD(P)H:quinone oxidoreductase activity in vitro. Here, we describe a protocol to measure oxidoreductase activity of Fld-LPs that are present in extracts of whole cells. We have recently shown that the assembly and activity of a Fld-LP, CgPst2, is regulated by an aspartyl protease-mediated cleavage of its C-terminus in the pathogenic yeast Candida glabrata. Mutant yeast where the CgPST2 gene was deleted lacked cellular NAD(P)H:quinone oxidoreductase activity and displayed elevated susceptibility to menadione stress. The protocol described herein is based on the measurement of NADH oxidation (conversion of NADH to NAD+) by endogenous Fld-LPs in the presence of quinone menadione. This assay can be performed with whole cell lysates prepared by the mechanical lysis of C. glabrata cells and does not require expression and purification of Fld-LPs from a heterogeneous system, thereby allowing researchers to study the effect of different posttranslational modifications and varied structural states of Fld-LPs on their enzymatic activities. Since many FLP-LPs are known to exist in dimeric and tetrameric states possessing differential activities, our efficient and easy-to-use assay can reliably detect and validate their quinone reductase activities. Although we have used menadione with CgPst2 enzyme in our study, the protocol can easily be modified to examine the presence of Fld-LPs with specificity for other quinones. As this assay does not require many expensive chemicals, it can readily be scaled up and adapted for other medically important fungi and potentially be a useful tool to characterize fungal oxidative stress response systems and screen inhibitors specific for fungal Fld-LPs, thereby contributing to our understanding of fungal pathogenesis mechanisms.
Keywords: Flavodoxin (黄素氧(化)还(原)蛋白)Background
Invasive fungal infections are a common cause of morbidity, mortality, and high healthcare costs in hospitals worldwide (Bongomin et al., 2017). Candida species are opportunistic human fungal pathogens, with Candida glabrata being the second to fourth most prevalent etiological agent of Candida bloodstream infections (Chakrabarti et al., 2015; Bongomin et al., 2017; Lamoth et al., 2018; Pfaller et al., 2019). Cells of the innate immune system play an important role in the control of Candida infections through an armamentarium of reactive oxygen and nitrogen species generation, antifungal peptide production, and facilitating fungal antigen presentation to activate the adaptive immune system (Netea et al., 2015; Heung, 2020). C. glabrata encounters reactive oxygen species (ROS)-induced stress upon internalization by host macrophages (Seider et al., 2011; Rai et al., 2012). However, it probably possesses a robust oxidative stress defense system that facilitates its survival and replication in macrophages (Kaur et al., 2007; Seider et al., 2011; Rai et al., 2012). Of note, macrophages are thought to act as Trojan horses for C. glabrata, providing it with a safe niche in the human host (Brunke and Hube, 2013).
Efficient detoxification of ROS is necessary to survive an oxidative environment. C. glabrata shows high levels of resistance towards oxidative stress caused by hydrogen peroxide in vitro (Cuéllar-Cruz et al., 2008). The sole catalase CgCta1 and two superoxide dismutases, CgSod1 and CgSod2, contribute to the effective oxidative stress response system in C. glabrata (Cuéllar-Cruz et al., 2008; Briones-Martin-Del-Campo et al., 2015). Another arm of the oxidative stress defense system is represented by proteins containing flavodoxin-like fold that are implicated in quinone detoxification (Sancho, 2006; Carey et al., 2007; Lodeyro et al., 2012). The flavodoxin-fold is characterized by a 5-stranded parallel β-sheet surrounded by α-helices on both sides, and present in proteins that are involved in electron transfer between varied redox compounds (Sancho, 2006; Carey et al., 2007; Lodeyro et al., 2012; Farías-Rico et al., 2014; Houwman and van Mierlo, 2017). Many proteins with flavodoxin-like fold possess NAD(P)H:quinone oxidoreductase activity and detoxify quinones to hydroquinones by preventing the formation of unstable and highly reactive semiquinone species, and use FMN (flavin mononucleotide) or FAD (flavin adenine dinucleotide) as cofactors (Patridge and Ferry, 2006; Sancho, 2006; Carey et al., 2007; Farías-Rico et al., 2014; Koch et al., 2017). This enzymatic reaction follows a ping-pong bi-bi mechanism, occurs in two half-reactions, reductive and oxidative, and involves two direct hydride transfer steps (Deller, 2008). The reductive half-reaction involves electron donation by the reduced form of nicotinamide adenine dinucleotide, NADH, to cofactors FMN or FAD, with NADH getting oxidized to NAD+. In the oxidative half-reaction, the reduced cofactors, FMN(H2) or FAD(H2), transfer the electrons to quinone substrates, which leads to the reduction of quinones to hydroquinones (Deller, 2008).
We have recently identified and characterized four flavodoxin-like proteins (Fld-LPs) in C. glabrata: CgPst2, CgRfs1, CgPst3, and CgYcp4, and shown that CgPst2 is uniquely required to survive menadione and benzoquinone stress (Battu et al., 2021). Since the bacterially purified CgPst2 showed no NAD(P)H:quinone oxidoreductase activity (Battu et al., 2021), we developed a protocol to measure NAD(P)H:quinone oxidoreductase activity in whole cell lysates of wild-type and CgPST2 gene-deleted strains to establish the role of CgPst2 in quinone detoxification. The step-by-step procedure followed to carry out the assay is represented in Figure 1. Our protocol is based on the conserved ping-pong mechanism of flavodoxin-like proteins, wherein we monitored the oxidation of NADH to NAD+ at 340 nm as a measure of quinone conversion to quinol/hydroquinone. Since the reduced form of NADH absorbs at 340 nm, while the oxidized form NAD+ has no absorbance at 340 nm, NADH oxidation was recorded as a decrease in the absorbance at 340 nm. This reaction mechanism is schematically illustrated in Figure 2.
Using this assay, we were able to show that menadione treatment leads to an increase in cellular NAD(P)H:quinone oxidoreductase activity, which could be partially attributed to elevated CgPst2 levels and enhanced aspartyl protease CgYps1-mediated cleavage of CgPst2, stimulating homo-tetramerization of CgPst2 (Battu et al., 2021). Notably, purified Fld-LPs WrbA and Pst2 exhibited NAD(P)H:quinone oxidoreductase activity in Escherichia coli and Saccharomyces cerevisiae, respectively (Patridge and Ferry, 2006; Koch et al., 2017). However, using the protocol developed, we could show NAD(P)H:quinone oxidoreductase activity in whole cell extracts of C. glabrata leading to NADH oxidation in the presence of menadione in vitro (Battu et al., 2021). Importantly, this activity was absent in strains deleted for the CgPST2 gene, and the activity was regained upon expression of CgPST2 gene in mutant strains (Battu et al., 2021), thereby unequivocally correlating the cellular NAD(P)H:quinone oxidoreductase activity with the presence of CgPst2 in cells.
The advantages of our protocol are many-fold. First, it is an efficient way to measure NAD(P)H:quinone oxidoreductase activity in cellular extracts and does not require cloning, expression, and purification of Fld-LPs in a heterologous expression system. Second, it neither requires expensive chemicals nor sophisticated equipment. Third, our assay is easy-to-use and can readily be applied to study different quinone detoxification activities under varied stress and host-mimicking conditions, as well as in mutants lacking various genes of the oxidative stress response machinery and yeast cells recovered from host immune cells, including macrophages. It can also be adapted to screen inhibitors that are specific for fungal Fld-LPs. Fourth, unlike activity measurement in purified enzymes, no additional supply of FMN/FAD is required as cofactor in our assay. Finally, since this protocol estimates NAD(P)H:quinone oxidoreductase activity in cellular extracts, the loss of enzymatic activity often observed during protein purification is avoided. Thus, this assay can provide key insights into the activity of Fld-LPs present in their native states, including Fld-LPs containing different posttranslational modifications and/or oligomeric Fld-LPs.
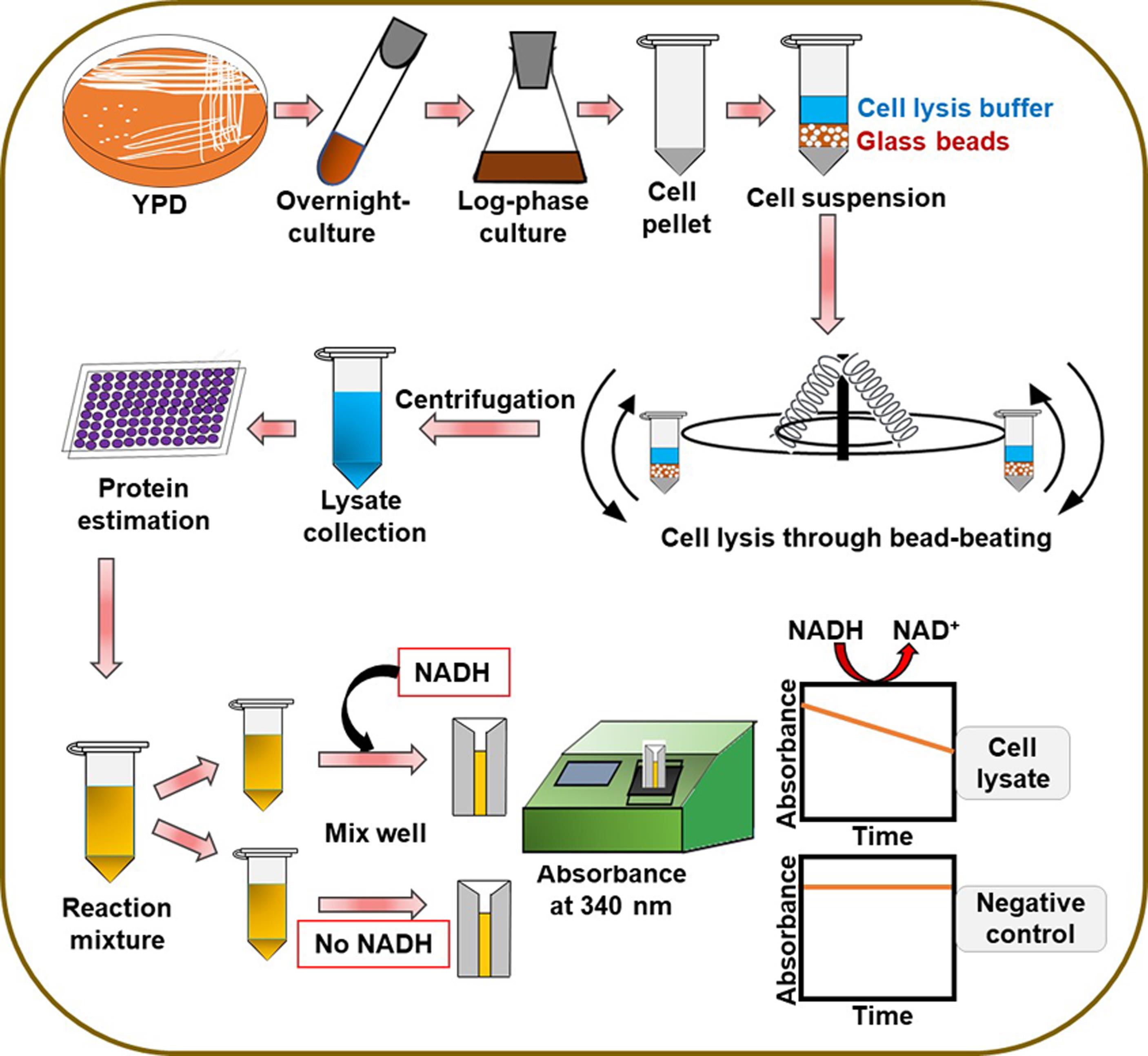
Figure 1. Schematic representation of the steps in the protocol
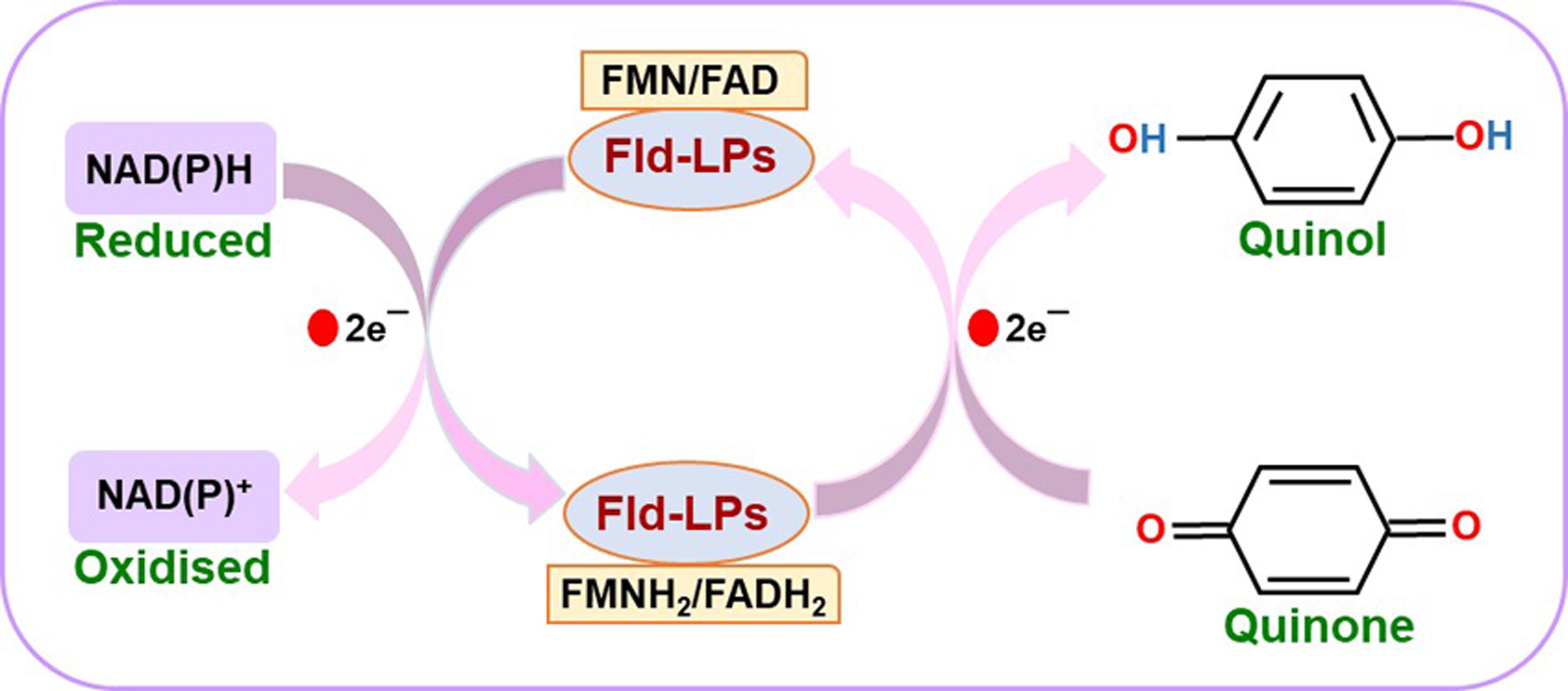
Figure 2. Schematic illustration of the reaction mechanism of flavodoxin-like proteins (Fld-LPs)
Materials and Reagents
40 ml glass test tube
Cotton wads
1.5 ml centrifuge tubes (Tarsons, catalog number: 500010)
15 ml Falcon tubes (Tarsons, catalog number: 546021)
0.5 mm diameter glass beads (BioSpec Products, catalog number: 11079105)
96-well plates (Biofil, catalog number: 011096)
Menadione (Sigma-Aldrich, catalog number: M5625)
Ethanol (PHARMCO-AAPER, catalog number: 111WORLDUV200)
200 mM phenylmethylsulphonyl fluoride (PMSF, serine proteases inhibitor) (Amresco, catalog number: 0754-5G)
100 mM sodium orthovanadate (NaOVa, Tyr and alkaline phosphatase inhibitor) (Sigma-Aldrich, catalog number: S6508)
200 mM sodium fluoride (NaF, Ser/Thr and acid phosphatase inhibitor) (Sigma-Aldrich, catalog number: 450022)
100× protease inhibitor cocktail (Sigma-Aldrich, catalog number: P8215)
PierceTM BCA protein assay kit (Thermo Scientific, catalog number: 23227)
Nicotinamide adenine dinucleotide (NADH; reduced form of NAD), disodium salt, grade 2 approx. 98% (500 µM) (Roche, catalog number: 10128023001)
NAD(P)H: FMN oxidoreductase from Photobacterium fischeri (Roche, catalog number: 10476480001)
Culture media: Yeast extract (1%)-Peptone (2%)-Dextrose (2%) YPD (Becton, Dickinson and Company (BD) Diagnostic Systems, catalog number: 242810) (see Recipes)
Phosphate Buffer Saline (PBS) (see Recipes)
NaCl (Affymetrix, catalog number: 21618)
KCl (G-Biosciences, catalog number: RC1167)
Na2HPO4 (Fisher Scientific, catalog number: 27785)
KH2PO4 (Fisher Scientific, catalog number: 13405)
Cell lysis buffer (see Recipes)
Tris base (MP Biosciences, catalog number: 808229)
EDTA (Sigma-Aldrich, catalog number: E6635)
Dextrose (Becton, Dickinson and Company (BD) Biosciences, catalog number: 215530)
Quinone reductase activity assay buffer (see Recipes)
Tris base (MP Biosciences, catalog number: 808229)
NaCl (Affymetrix, catalog number: 21618)
HPLC grade water (Merck Life Science Pvt. Limited, catalog number: 61765010001730)
Equipment
30°C shaker incubator (New Brunswick Innova, model: 43R)
Spectrophotometer/Cell density meter (Biowave-WFA, model: C08000)
Fine weighing balance (Mettler Toledo, model: MS205DU)
FastPrep 24 homogenizer or Bead beater (MP Biomedicals, model: 19083976)
Cooling table top centrifuge (Eppendorf, model: 5424R)
Quartz cuvette, 10 mm light path (Hellma Analytics, catalog number: 104-10-40)
SpectraMax multimode microplate reader (Molecular Devices, model: M5)
Needle and Spatula (Local stores)
Software
Excel 2018 (Microsoft)
SoftMax Pro Software 7.0
GraphPad Prism 7
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Battu, A., Purushotham, R. and Kaur, R. (2021). An Assay to Determine NAD(P)H: Quinone Oxidoreductase Activity in Cell Extracts from Candida glabrata. Bio-protocol 11(21): e4210. DOI: 10.21769/BioProtoc.4210.
分类
微生物学 > 微生物生物化学 > 蛋白质 > 活性
微生物学 > 微生物生理学
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link