- EN - English
- CN - 中文
Thermal Proteome Profiling to Identify Protein-ligand Interactions in the Apicomplexan Parasite Toxoplasma gondii
弓形虫热蛋白质组分析以识别顶复体寄生虫中的蛋白质-配体相互作用
发布: 2021年11月05日第11卷第21期 DOI: 10.21769/BioProtoc.4207 浏览次数: 6171
评审: John P PhelanKristin L. ShinglerAnonymous reviewer(s)
Abstract
Toxoplasma gondii is a single-celled eukaryotic parasite that chronically infects a quarter of the global population. In recent years, phenotypic screens have identified compounds that block parasite replication. Unraveling the pathways and molecular mechanisms perturbed by such compounds requires target deconvolution. In parasites, such deconvolution has been achieved via chemogenomic approaches—for example, directed evolution followed by whole-genome sequencing or genome-wide knockout screens. As a proteomic alternative that directly probes the physical interaction between compound and protein, thermal proteome profiling (TPP), also known as the cellular thermal shift assay (CETSA), recently emerged as a method to identify small molecule–target interactions in living cells and cell extracts in a variety of organisms, including unicellular eukaryotic pathogens. Ligand binding induces a thermal stability shift—stabilizing or destabilizing proteins that change conformationally in response to the ligand—that can be measured by mass spectrometry (MS). Cells are incubated with different concentrations of ligand and heated, causing thermal denaturation of proteins. The soluble protein is extracted and quantified with multiplexed, quantitative MS, resulting in thousands of thermal denaturation profiles. Proteins engaging the ligand can be identified by their compound-dependent thermal shift. The protocol provided here can be used to identify ligand-target interactions and assess the impact of environmental or genetic perturbations on the thermal stability of the proteome in T. gondii and other eukaryotic pathogens.
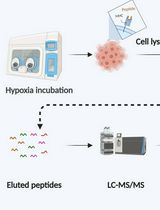
Graphic abstract:

Thermal proteome profiling for target identification in the apicomplexan parasite T. gondii.
Background
Target deconvolution is a major challenge for the wealth of compounds identified through phenotypic screening. Chemogenomic approaches, such as directed evolution or drug screens, have been the favored tools for target identification in eukaryotic parasites (Paquet et al., 2017; Cowell et al., 2018; Luth et al., 2018; Rosenberg et al., 2019; Harding et al., 2020). Such approaches require culturing parasites and host cells under compound treatment for extended periods and often identify pathways indirectly affected by a small molecule rather than the target itself. In contrast, several proteomic methods developed in the past decade directly identify interactions between compounds and protein targets (McClure and Williams, 2018; Conway et al., 2021). For example, enrichment of interacting proteins can be performed with derivatized compounds for affinity-purification followed by mass spectrometry (MS). However, these approaches require specialized chemistry and introduce a linker and other chemical groups to the compound of interest, which may affect its behavior.
Thermal proteome profiling (TPP), also known as the cellular thermal shift assay (CETSA), offers a label-free approach that can be performed in a variety of formats that preserve cellular physiology, including in situ (Dai et al., 2019; Mateus et al., 2020b). Interactions with a target are identified by a compound-dependent shift in the protein’s thermal profile. Cells or cell extracts are treated with the compound and heated to induce thermal denaturation. Aggregated proteins are removed, and soluble proteins are quantified by MS to generate melting curves for each protein. TPP has recently identified the targets of antiparasitic compounds in the apicomplexan parasites Plasmodium falciparum (Dziekan et al., 2019; Lu et al., 2020) and Toxoplasma gondii (Herneisen et al., 2020), as well as in the trypanosome Leishmania donovani (Corpas-Lopez et al., 2019).
The application of TPP extends beyond target deconvolution (Becher et al., 2018; Dai et al., 2018 and 2019; Sridharan et al., 2019; Mateus et al., 2020b). Alterations to protein state and stability may arise from conformational changes, post-translational modifications, altered localization, and interactions with other proteins and biomolecules such as metabolites and nucleic acids. For example, we performed TPP on parasites lacking mitochondrial DegP2 to identify proteins with altered stability based on the loss of this protease (Harding et al., 2020). Genetic perturbations in conjunction with functional proteome profiling are in the early stages (Mateus et al., 2020a) and may be especially well-suited to map the unannotated parts of parasite proteomes.
While TPP has been performed predominantly in mammalian systems, it is expanding to other organisms (Corpas-Lopez et al., 2019; Dziekan et al., 2019; Volkening et al., 2019; Lu et al., 2020; Herneisen et al., 2020; Harding et al., 2020; Jarzab et al., 2020). We believe this approach pairs particularly well with the study of eukaryotic parasites, whose evolutionary divergence complicates identifying molecular pathways by genomic annotation or bioinformatic analysis. For that reason, we provide a detailed protocol describing our thermal profiling pipeline developed for the organism T. gondii. Below, we identify key considerations for selecting a TPP workflow appropriate for the researcher’s biological question. Step-by-step guidelines follow.
Types of experiment
In this protocol, we stratify steps by choice of material and treatment. TPP can be performed on live parasites or parasite lysates and by melting samples over a range of 10 temperatures (“temperature range”) or over a range of 10 compound concentrations melted at a single temperature (“concentration range”). These variations give rise to four permutations described in the Procedure as (B) Lysate Temperature Range Experiment, (C) Parasite Temperature Range Experiment, (D) Lysate Concentration Range Experiment, and (E) Parasite Concentration Range Experiment. Each experiment has advantages and disadvantages (Franken et al., 2015; Dai et al., 2019; Mateus et al., 2020b). For example, experiments using live cells are more physiological but combine direct and indirect effects. Lysate experiments may more directly identify ligand-protein interactions, but loss of cellular compartmentalization can also lead to non-physiological interactions. Concentration range experiments yield more information-rich thermal profiles, but real interactions may be missed if the thermal challenge temperatures are suboptimal (too low or too high) and the overall coverage of the proteome is reduced due to global denaturation.
Treatment conditions
We have performed thermal profiling experiments on extracellular parasites to avoid the added complexity of the host proteome and confounding effects from compound permeability and host metabolism. Compound treatments are often performed on intracellular parasites; however, the appropriate concentration of compound for the thermal profiling experiment should be determined by assays using extracellular parasites. The thermal profiling experiment should mimic assay conditions as closely as possible. Considerations include the amount of time needed for the compound to arrive at diffusion and binding equilibria, the buffer in which the equilibration takes place, and equilibration temperature (e.g., room temperature vs. 37°C). Mammalian studies have often performed the incubation in PBS (Reinhard et al., 2015; Savitski et al., 2014; Franken et al., 2015). We have used a buffer composition resembling the ionic makeup of the host cytosol (Herneisen et al., 2020; Harding et al., 2020). Buffers should lack serum, which would overwhelm parasite signals that can be quantified by MS.
We aim to process 25 µg of protein per reference sample, which in our experience corresponds to the material from 1 × 107 extracellular parasites of the type I RH strain. We subject concentration-range samples to at least two different thermal challenge temperatures; we have found 54°C and 58°C to work well while still providing sufficient coverage of the proteome. The thermal challenge temperatures may need to be optimized for each experiment; further commentary is provided in the Data analysis section.
Lysis conditions
The final lysis buffer composition should contain 0.5-1% IGEPAL CA-630 (also known as NP-40), which provides a balance between solubilizing membrane proteins without re-solubilizing aggregated proteins (Reinhard et al., 2015). For most experiments, the lysis buffer should contain protease inhibitors (and optionally phosphatase inhibitors, depending on the focus of the experiment) and benzonase for digestion of nucleic acids prior to the SP3 cleanup. If the compound of interest is thought to affect proteases, phosphatases, or nucleic acid binding activity, then these supplements should be omitted until after the Separation of Soluble and Aggregated Protein. Our lysis buffers have had an ionic composition similar to PBS (Herneisen et al., 2020) and an intracellular-like buffer (Harding et al., 2020), depending on the application. The ionic composition of the buffer (e.g., presence of ATP and metabolites) can substantially influence the melting behavior of proteins (Lim et al., 2018; Sridharan et al., 2019). The concentration of parasite lysate also influences melting behavior; therefore, it is crucial to count the number of parasites prior to lysis and use a consistent lysis buffer volume for the number of parasites. Following harvest, parasites should be resuspended at least once in a wash buffer that is similar in composition to the lysis buffer (but lacking detergents) to dilute cell culture contaminants, such as serum proteins.
Materials and Reagents
T12.5 flask (e.g., Corning Falcon Tissue Culture Flasks, catalog number: 29185-298)
T175 flask (e.g., CELLSTAR® Filter Cap Cell Culture Flasks, catalog number: 82050-872)
15-cm dish (e.g., Corning Falcon® Tissue Culture Dishes, catalog number: 25383-103)
Corning® 150 ml Bottle Top Vacuum Filter, 0.22 µm Pore 13.6 cm2 CA Membrane (Corning, catalog number: 430624)
50 ml conical tube (Corning, catalog number: 430829)
Human foreskin fibroblast (HFF) cells (ATCC, catalog number: SCRC-1041)
T. gondii cell lines (RH, e.g., ATCC 50838 or PRA-319)
T. gondii filter (Whatman Pop-Top and Swin-Lok Plastic Filter Holders for 47 mm membrane filter size, e.g., VWR catalog number: 28163-089, with GE Healthcare Whatman Nuclepore Hydrophilic Membrane 3 or 5 µm circles, catalog number: 111112 or 111113)
Cell scraper (Corning® Small Cell Scraper, catalog number: 3010)
Protein low-bind tube (e.g., EppendorfTM LoBind Microcentrifuge Tubes, 1.5 ml Thermo Fisher Scientific, catalog number: 13698794)
8-strip PCR tubes (e.g., Genesee Scientific, catalog number: 27.125 U)
Thickwall polycarbonate open-top ultracentrifuge tubes (0.2 ml, 7 × 20 mm; Beckman Coulter, catalog number: 343775)
Protein low-bind 96-well plate (Eppendorf, catalog number: 951032905)
Syringes 20 ml (BD Biosciences, catalog number: 302830)
Hydrophobic Sera-Mag Speed Beads (GE Healthcare, catalog number: 65152105050250, ~50 mg/ml, keep at 4°C until use)
Hydrophilic Sera-Mag Speed Beads (GE Healthcare, catalog number: 45152105050250, ~50 mg/ml, keep at 4°C until use)
DMEM (Thermo Fisher Scientific, catalog number: 11965118, keep at 4°C until use)
Newborn Calf Serum USA origin, heat Inactivated, sterile-filtered, suitable for cell culture (Sigma-Aldrich, catalog number: N4762-500ML, keep at -80°C until use)
10 mg/ml gentamicin (Life Technologies, catalog number: 15710072, room temperature)
200 mM L-glutamine (Life Technologies, catalog number: 25030081, keep at -20°C until use)
250 U/µl benzonase (Sigma-Aldrich, catalog number: E1014-25KU, store at -20°C)
100× Halt Protease Inhibitor Cocktail (Life Technologies, catalog number: 87786)
IGEPAL® CA-630 viscous liquid (Sigma-Aldrich, catalog number: I3021-50ML)
10× PBS suitable for tissue culture (e.g., VWR, catalog number: 45001-130)
DC Protein Assay (Bio-Rad, catalog number: 5000116)
Tris(2-carboxyethyl)phosphine (TCEP; Pierce, catalog number: 20490; keep at -20°C until use)
Methyl methanethiosulfonate (MMTS; Thermo Fisher Scientific, catalog number: 23011, keep at 4°C)
Ethyl alcohol, Pure 200 proof, HPLC/spectrophotometric grade (Sigma-Aldrich, catalog number: 459828-1L)
Sequencing-grade trypsin (e.g., Promega, catalog number: V5113, keep at -80°C until use)
Triethylammonium bicarbonate buffer 1.0 M, pH 8.5 (Sigma-Aldrich, catalog number: T7408-100ML, keep at 4°C)
Pierce Quantitative Fluorometric Peptide Assay (Thermo Fisher Scientific, catalog number: 23290, keep at 4°C until use)
TMT10plex Isobaric Label Reagent Set (Thermo Fisher Scientific, catalog number: 90110, keep at -20°C until use)
50% hydroxylamine (Thermo Fisher Scientific, catalog number: 90115)
Pierce high pH fractionation kit (Thermo Fisher Scientific, catalog number: 84868, keep at 4°C until use)
Ultra-high-performance liquid chromatography (UPLC)-MS acetonitrile (Thermo Fisher Scientific, catalog number: A9561)
UHPLC-MS water (Thermo Fisher Scientific, catalog number: W81)
Pierce Formic Acid, LC-MS Grade (Thermo Fisher Scientific, catalog number: 28905)
DMEM + 3% CFS (see Recipes)
PBS (see Recipes)
10% IGEPAL CA-630 (also known as NP-40) (see Recipes)
10× CETSA buffer (see Recipes)
CETSA wash buffer (see Recipes)
CETSA lysis buffer (see Recipes)
1 M TCEP stock solution (see Recipes)
200 mM MMTS stock solution (see Recipes)
Buffer A (see Recipes)
Buffer B (see Recipes)
Equipment
CO2 incubator (Thermo Fisher Scientific Forma Steri-Cycle 370, catalog number: 370)
Clinical benchtop centrifuge (Eppendorf, model: Centrifuge 5810R, catalog number: 022625101)
Microcentrifuge (Eppendorf, model: Centrifuge 5424R [discontinued], alternatives include Centrifuge 5425/5425 R)
Minicentrifuge (VWR Galaxy Mini Centrifuge, catalog number: 37000-700)
Hemocytometer (VWR Counting Chamber, catalog number: 1517O-173)
Thermal cyclers (Bio-Rad C1000 TouchTM Thermal Cycler with Dual 48/48 Fast Reaction Module, catalog number: 1851148 and Bio-Rad C1000 TouchTM Thermal Cycler with 96-Deep Well Reaction Module, catalog number: 1851197)
Benchtop ultracentrifuge (Beckman Ultra MAX [discontinued], alternatives include the Optima MAX-XP and Optima MAX-TL)
Thermo mixer (Eppendorf, model: ThermoMixer C, catalog number: 5382000023 with 1.5 ml SmartBlock, catalog number: 5360000038)
Magnetic stand (Invitrogen Dynamag 2, catalog number: 12321D)
Vacuum centrifuge (SavantTM Universal SpeedVacTM Vacuum System, catalog number: SPD111V and, catalog number: UV5450)
Lyophilizer (Labconco FreeZone Triad Freeze Dryer, catalog number: 794001030)
Orbitrap mass spectrometer (Thermo Fisher Scientific Q Exactive HFX [discontinued] or Exploris 480, catalog number: BRE725533) with optional FAIMS Pro Interface (Thermo Fisher Scientific, catalog number: FMS02-10001)
MS-coupled LC system (Thermo Fisher Scientific EASY-nLC 1200, catalog number: LC140) with Acclaim PepMap 100 75 µm × 2 µm nanoViper trapping column (Thermo Fisher Scientific, catalog number: 164946) and PepMap RSLC C18 3 µm, 100A, 75 µm × 15 cm analytical column (Thermo Fisher Scientific, catalog number: ES900)
Pierce formic acid, LC-MS grade (Life Technologies, catalog number: 28905)
UPLC-MS acetonitrile (Thermo Fisher Scientific, catalog number: A9561)
UPLC-MS water (Thermo Fisher Scientific, catalog number: W81)
Software
Proteome Discoverer, version 2.4 (Thermo Fisher Scientific)
R, version 4.0 or later: https://cran.r-project.org/
Tidyverse package, version 1.3: https://cran.r-project.org/web/packages/tidyverse/index.html
TPP package, release 3.12: https://bioconductor.org/packages/TPP/
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Herneisen, A. L. and Lourido, S. (2021). Thermal Proteome Profiling to Identify Protein-ligand Interactions in the Apicomplexan Parasite Toxoplasma gondii. Bio-protocol 11(21): e4207. DOI: 10.21769/BioProtoc.4207.
分类
生物化学 > 蛋白质 > 定量
微生物学 > 微生物蛋白质组学 > 全生物体
药物发现
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link