- EN - English
- CN - 中文
High-throughput 3D Spheroid Formation and Effective Cardiomyocyte Differentiation from Human iPS Cells Using the Microfabric Vessels EZSPHERETM
利用微细血管EZSPHERETM从人类iPS细胞中进行高通量三维球状细胞形成和有效的心肌细胞分化
发布: 2021年11月05日第11卷第21期 DOI: 10.21769/BioProtoc.4203 浏览次数: 3799
评审: Alessandro DidonnaFereshteh AzediAnonymous reviewer(s)

相关实验方案
人 iPSC 衍生神经元与少突胶质细胞共培养用于髓鞘形成的小分子筛选分析
Stefanie Elke Chie [...] Maria Consolata Miletta
2025年05月05日 3401 阅读
Abstract
High-throughput 3D spheroid formation from human induced pluripotent stem cells (hiPSCs) can be easily performed using the unique microfabric vessels EZSPHERE, resulting in effective and large scale generation of differentiated cells such as cardiomyocytes or neurons. Such hiPSC-derived cardiomyocytes (hiPSC-CMs) or neurons are very useful in the fields of regenerative medicine or cell-based drug safety tests. Previous studies indicated that 3D spheroids arising from hiPSCs are effectively differentiated into high quality hiPSC-CMs by controlling Wnt signals through utilization of the microfabric vessels EZSPHERE. Here, we describe a simple and highly efficient protocol for generating a large number of uniformly sized hiPSC spheroids and inducing them for cardiac differentiation using the EZSPHERE. This method comprises the collection and dissociation of the spheroids from cardiac differentiation medium, in the middle stage of the whole cardiac differentiation process, and re-seeding the obtained single cells into the EZSPHERE to re-aggregate them into uniform hiPSC-CM spheroids with controlled size. This re-aggregation process promotes non-canonical Wnt signal-related cardiac development and improves the purity and maturity of the hiPSC-CMs generated.
Graphic abstract:
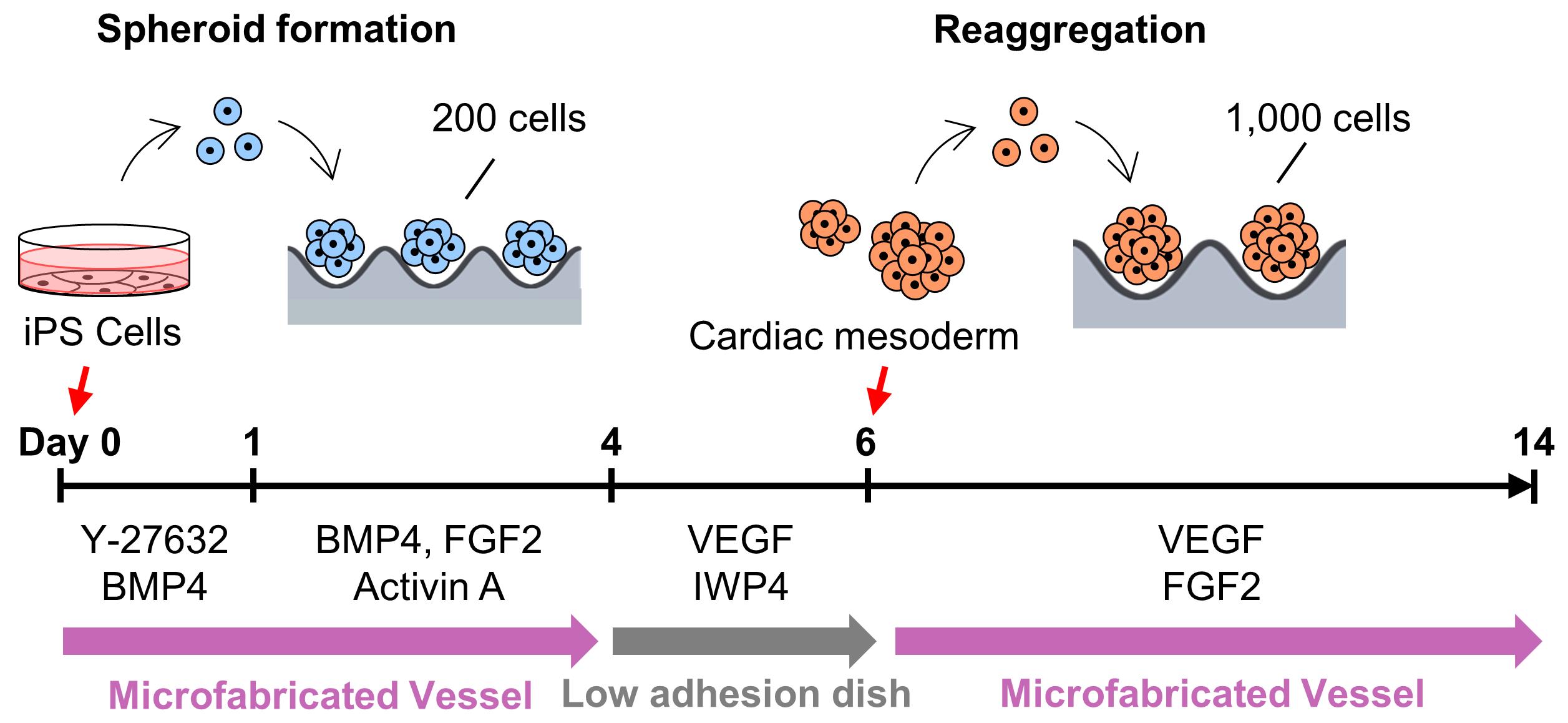
Overview of cardiac differentiation from iPSCs by spheroid formation and reaggregation using EZSPHERE.
Background
Human induced pluripotent stem cell-derived Cardiomyocytes (hiPSC-CMs) are expected to become a cell source for applications in regenerative medicine of heart diseases and drug discovery. Heart diseases remain the leading cause of death worldwide (WHO, 2018). In non-human primates, transplanted hiPSC-CMs improve cardiac contraction function and are sufficient to regenerate the infarcted heart (Shiba et al., 2016). Previous studies, represented by the CiPA Project (Blinova et al., 2017) and the JiCSA Project (Yamazaki et al., 2018), have shown the utility of hiPSC-CMs for prediction of the risk of cardiotoxicity, like Torsades de Pointes. It was also shown that maturity of the generated hiPSC-CMs alters the response to drugs (da Rocha et al., 2017). Thus, the development of a high-throughput and robust protocol for generating high-quality hiPSC-CMs is urgent for the realization of regenerative medicine and drug discovery.
hiPSCs could be directly differentiated into cardiomyocytes in either 2D adhesion culture or 3D cell aggregates/spheroid culture methods. In particular, the spheroid culture method is easier to scale-up and better at promoting maturation of the generated hiPSC-CMs, compared with the 2D culture method (Pal et al., 2013). In the cardiac differentiation process, regulation of canonical and non-canonical Wnt at the right time strongly promotes pluripotent stem cell differentiation into cardiomyocytes, not other mesodermal cell types (Loh et al., 2016). In addition, expression levels of the Wnt signal-related genes Wnt5A and Wnt11 are affected by spheroid size (Hwang et al., 2009, Chen et al., 2015). Therefore, we sought to develop a highly efficient and robust cardiac differentiation protocol by achieving high-throughput spheroid formation with simultaneous active spheroid size control.
In this study, we used the uniquely shaped microfabric dishes, “EZSPHERE,” designed for high-throughput production of uniform spheroids on one culture dish, with its high-density micro-wells (Sato et al., 2016). Initially, a basic spheroid culture protocol, optimized for cardiac differentiation (Yang et al., 2008, Bauwens et al., 2014, Kempf et al., 2014), was combined with the EZSPHERE dishes. For spheroid formation from the hiPSCs, Type 900 EZSPHERE dishes with a diameter of 100 mm were used. Each of them had approximately 14,000 individual micro-wells with regular size (400-500 μm in diameter and approximately 100 μm in depth). On calculation, approximately 14,000 spheroids could be formed in each EZSPHERE dish, and their initial seeding cell number per spheroid ranged 200-220. As per standard protocol, activin A, BMP-4, and FGF2 were added to the spheroids in the EZSPHERE to induce differentiation into Primitive Streak on day 1. On day 4, the spheroids were collected, washed, and transferred onto a flat bottom low cell adhesion dish, followed by treatment with Wnt inhibitor. On day 6, the size uniformity of the spheroids was lost, but 62% of the cell population had differentiated into PDGFR-α/KDR double-positive cardiac mesoderm-like cells (Kattman et al., 2011; Birket et al., 2015). To control spheroid size once more, the differentiated spheroids were collected and dissociated into single cells. Then, the single cells obtained were re-aggregated by re-seeding onto the dishes of Type 903 EZSPHERE (with a diameter of 35 mm), which have approximately 1,000 individual micro-wells with larger size (approximately 800 μm diameter and 400 μm depth) in each dish. The re-aggregated spheroids increased the number of cardiac Troroponin T (CTNT) positive cells faster than that of the non-reaggregated spheroids. In addition, the cardiac maturity (estimated by the gene expression level of MYL2 per MYL7) was much higher in the re-aggregated spheroids compared with that of the spheroids that did not undergo the re-aggregation process. Performing the re-aggregation process also raised the expression levels of the non-canonical Wnt signals Wnt5A and Wnt11. In mice, Wnt5A and Wnt11 are essential for the generation of second heart field progenitor cells (Cohen et al., 2012). Wnt11 is also known for its role in generating myocardial electrical gradient patterns through the regulation of non-canonical Wnt/Ca2+ signaling during zebrafish heart development (Panakova et al., 2010). To reveal the detail of its molecular mechanism, further study is required. However, these data strongly suggest that the cardiomyocyte differentiation process performed on EZSPHERE, including a re-aggregation step for the induced cardiac mesoderm/progenitor, is effective in producing cardiomyocytes with high purity and maturation levels.
Materials and Reagents
EZSPHERE Dish 100 mm Type 900 (AGC Techno Glass, IWAKI, catalog number: 4020-900)
EZSPHERE Dish 35 mm Type 903 (AGC Techno Glass, IWAKI, catalog number: 4000-903)
EZ-BindShut II 100 mm Dish (AGC Techno Glass, IWAKI, catalog number: 4020-800LP)
Cell culture T25 Flask (AGC Techno Glass, IWAKI, catalog number: 3100-025)
5 ml Pipet (AGC Techno Glass, IWAKI, catalog number: 7103-005)
Cell scraper (AGC Techno Glass, IWAKI, catalog number: 9010-320)
70 μm cell strainer (Corning, Falcon, catalog number: 352350)
Pasteur pipettes (AGC Techno Glass, IWAKI, catalog number: IK-PAS-9P)
Human induced pluripotent stem cell (hiPSC) strains 253G1 and 201B7 (iPS Academia Japan)
Maintenance culture media mTeSR1 (Stemcell Technologies, catalog number: 5850)
Biolaminin 521 LN (LN521) (BioLamina, catalog number: LN521-03)
DPBS (FUJIFILM Wako Pure Chemical, Wako, catalog number: 045-29795)
HBSS, no calcium (Thermo Fisher Scientific, Gibco, catalog number: 14170112)
DMEM (4.5g/L Glucose) with L-Gln, without Sodium Pyruvate, liquid (Nacalai, catalog number: 08459-35)
0.5 mol/L-EDTA solution (pH 8.0) (Nacalai, catalog number: 06894-14)
Fetal bovine serum, FBS (MP Biomedicals, CELLECT, catalog number: 2916154)
Accutase (Merck, Sigma-Aldrich, catalog number: A6964-100ML)
AccuMax (Innovative Cell Technologies, catalog number: AM105)
TrypLE select Enzyme (1×), no phenol red (Thermo Fisher Scientific, Gibco, catalog number:12563011)
StemProTM-34 SFM (1×) (Thermo Fisher Scientific, Gibco, catalog number: 10639011)
Penicillin-Streptomycin (10,000 U/ml) (Thermo Fisher Scientific, Gibco, catalog number: 15140122)
L-Glutamine (200 mM) (Thermo Fisher Scientific, Gibco, catalog number: 25030081)
holo-Transferrin human (Merck, Sigma-Aldrich, catalog number: T0665-100MG)
L-Ascorbic acid (Merck, Sigma-Aldrich, catalog number: A92902-100MG)
1-Tioglycerol (Merck, Sigma-Aldrich, catalog number: M6145-25ML)
ROCK inhibitor Y-27632 (Wako Pure Chemical Industries, Wako, catalog number: 253-00513)
Recombinant Human BMP-4 Protein (Bio-Techne, R&D Systems, catalog number: 314-BP-010)
Fibroblast Growth Factor (basic), Human, recombinant (154 aa) (FUJIFILM Wako Pure Chemical, Wako, catalog number: 064-04541)
Recombinant Human VEGF 165 Protein (Bio-Techne, R&D Systems, catalog number: 293-VE-010)
Recombinant Human/Mouse/Rat Activin A Protein (Bio-Techne, R&D Systems, catalog number: 338-AC-010)
Stemolecule Wnt Inhibitor IWP-4 (REPROCELL USA, Stemgent, catalog number: 04-0036)
DNase I, Bovine Pancreas (Merck, Millipore, catalog number: 260913)
30 w/v% Albumin D-PBS (-) Solution, from Bovine Serum (BSA), Fatty Acid Free (FUJIFILM Wako Pure Chemical, Wako, catalog number: 015-23871)
4% Paraformaldehyde Phosphate Buffer Solution (Wako Pure Chemical Industries, Wako, catalog number: 163-20145)
Antibodies used in flow cytometry analysis (Table 1)
Table 1. Antibodies used in flow cytometry analysis
Antibodies Distributor Cat. No. Dilution ratio APC-KDR BioLegend 359915 2.5:100 APC-Mouse IgG1, kappa Isotype control BioLegend 400121 2.5:100 PE-PDGFRα BioLegend 323505 5:100 PE-Mouse IgG1, kappa Isotype control BioLegend 400113 5:100 PE anti-cardiac Troponin T BD Pharmingen 564767 2.5:100 PE-Isotype control BD Pharmingen 554680 2.5:100 Cell culture Reagents (see Recipes)
Cytokines and small molecular compounds (see Recipes)
Modified StemPro-34 (see Recipes)
Spheroid formation medium (see Recipes)
Stage-1 medium (2×) (see Recipes)
Stage-2 medium (see Recipes)
Stage-3 medium (see Recipes)
Equipment
Laminar flow hood (Hitachi, model: SCV-Class II type A/B)
Water bath (AS ONE Corporation, model: TR-1AR)
TC10 Automated Cell Counter (Bio-Rad, catalog number: 145-0001)
EVOS FL Auto Cell Imaging System (Life Technologies, EVOS, catalog number: AMAFD1000)
EVOS Onstage Incubator (Life Technologies, EVOS, catalog number: AMC1000)
BD FACSVerse flow Cytometer (BD Bioscience, catalog number: 651154)
Pipet-Aid XPress 110V (Drummond Scientific Company, catalog number: 4-040-135)
Centrifuge (Himac, CF16RX, model: S101812)
Vortex mixer Vortex Gwnie 2 (Scientific Industries, Inc., catalog number: SI-0236)
HERAcell CO2 incubator (Kendro Laboratory Products)
Vacuum pump (Model JN726 FT.18)
Software
ImageJ (NIH, https://imagej.nih.gov/ij/)
AGDRec AG-Desktop recorder (AmuseGraphics, http://t-ishii.la.coocan.jp/hp/ag/index.html)
FlowJo software (FlowJo LLC, https://www.flowjo.com/)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Miwa, T., Idiris, A. and Kumagai, H. (2021). High-throughput 3D Spheroid Formation and Effective Cardiomyocyte Differentiation from Human iPS Cells Using the Microfabric Vessels EZSPHERETM. Bio-protocol 11(21): e4203. DOI: 10.21769/BioProtoc.4203.
分类
干细胞 > 多能干细胞 > 细胞分化
细胞生物学 > 细胞分离和培养 > 细胞分化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link