- EN - English
- CN - 中文
Simultaneous Monitoring Cytoplasmic Calcium Ion and Cell Surface Phosphatidylserine in the Necrotic Touch Neurons of Caenorhabditis elegans
秀丽隐杆线虫坏死触觉神经元胞质钙离子和细胞表面磷脂酰丝氨酸的同时监测
发布: 2021年10月20日第11卷第20期 DOI: 10.21769/BioProtoc.4187 浏览次数: 2620
评审: Ehsan KheradpezhouhAnonymous reviewer(s)
Abstract
Calcium ions trigger many cellular events, including the release of neurotransmitters at the synaptic terminal and excitotoxic cell death. Recently, we have discovered that a transient increase in the level of cytoplasmic Ca2+ triggers the exposure of phosphatidylserine (PS) on the surfaces of necrotic cells in the nematode Caenorhabditis elegans. PS serves as an “eat me” signal that attracts engulfing cells to engulf and degrade necrotic cells. During the above study, we developed a microscopic imaging protocol for real-time monitoring the levels of cytoplasmic Ca2+ and cell surface PS in Caenorhabditis elegans touch neurons. Previously, Ca2+ dynamics was monitored in neurons in Caenorhabditis elegans larvae in time periods ranging from milliseconds to seconds. Methods for monitoring Ca2+ dynamics for a relatively long period of time during embryonic development were not available, let alone for simultaneous monitoring Ca2+ and PS dynamics. The protocol reported here utilizes a deconvolution imaging system with an optimized experimental setting that reduces photo-damage and allows the proper development of embryos during the real-time imaging process. This protocol enables the simultaneous measurement of cytosolic Ca2+ and cell surface PS levels in necrotic touch neurons during embryonic development in a period longer than six hours. Our method provides an easy and sensitive approach to perform long-time Ca2+ and PS recording in living animals, simultaneously or individually. This protocol can be applied to study various cellular and developmental events that involve the dynamic regulation of Ca2+ and/or PS.
Keywords: Calcium ion (钙含量)Background
Cytosolic Ca2+ is an important factor for regulating various cellular events such as gene expression, neurotransmitter release at synaptic terminal sites, muscle contraction, and hormone secretion (Clapham, 2007). Excessive amounts of Ca2+ in the cytoplasm provoke excitotoxic necrosis, often seen in neurodegenerative diseases (Yamashima, 2004; Wojda et al., 2008), heart failures (Nakayama et al., 2007), and cancer (Danese et al., 2017). Recently, we have found that a transient increase in Ca2+ level in the cytoplasm of neurons undergoing necrosis is necessary for the exposure of phosphatidylserine (PS), which acts as an “eat me” signal to attract engulfing cells on the surfaces of necrotic cells in the nematode Caenorhabditis elegans (Furuta et al., 2021). In previous studies, in Caenorhabditis elegans larvae, Ca2+ dynamics was monitored in the cell bodies of mechanosensory neurons (touch neurons) with Cameleon, a genetically encoded calcium indicator (GECI) (Suzuki et al., 2003). Different generations of GCaMP, another series of GECIs, were also reported to monitor Ca2+ in the neuronal processes of different sensory neurons in the Caenorhabditis elegans larvae (Tian et al., 2009; Ghosh-Roy et al., 2010; Akerboom et al., 2012). These Ca2+ recording experiments were performed in Caenorhabditis elegans larvae, and recording time ranged from milliseconds to seconds. However, recording of Ca2+ signal in Caenorhabditis elegans for longer periods of time or during embryonic development has not been reported previously.
We study the necrosis and clearance of Caenorhabditis elegans touch neurons during embryogenesis. In Caenorhabditis elegans, dominant mutations in the DEG/ENaC sodium channel subunit MEC-4 induce the six touch neurons to undergo excitotoxic necrosis (Figure 1A) (Chalfie and Sulston, 1981; Driscoll and Chalfie, 1991). These necrotic neurons expose PS and are subsequently engulfed and digested by neighboring hypodermal cells (Li et al., 2015). Previously, we developed secreted fluorescence PS reporters by tagging MFG-E8, a secreted PS-binding protein, with fluorescent proteins such as mCherry, and expressing each reporter under the control of the dyn-1 promoter (Pdyn-1) (Li et al., 2015). The secreted MFG-E8::mCherry protein associates with PS exposed on cell surfaces and, in this manner, labels the surfaces of necrotic and apoptotic cells (Figure 1B). We established a time-lapse recording protocol that allowed us to monitor, for hours, the dynamics of PS externalization on the surface of necrotic cells in Caenorhabditis elegans embryo (Li et al., 2015).
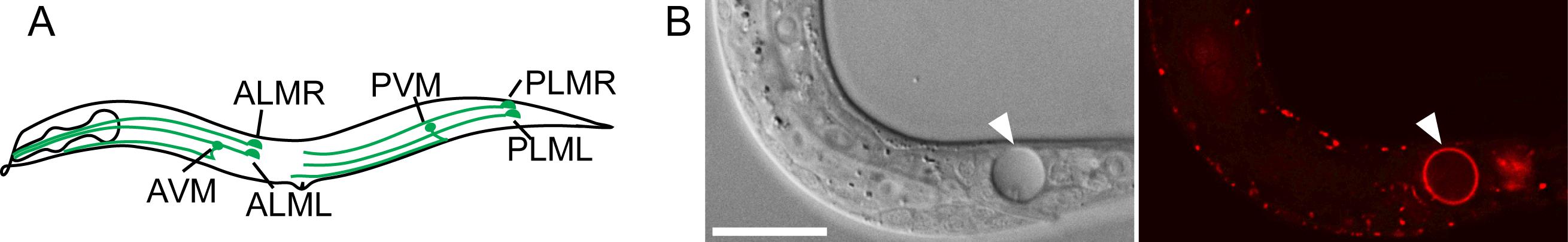
Figure 1. Caenorhabditis elegans six touch neurons and PS exposed on the surface of a necrotic cell. (A) Diagram of a hermaphrodite indicating the positions and names of the six touch neurons. (B) A necrotic cell caused by mec-4(e1611) mutation in Differential Interference Contrast (DIC) image (left) and exposed PS visualized by MFG-E8::mCherry reporter (right). The scale bar is 15 µm.
To investigate the effect of cytoplasmic Ca2+ in triggering PS exposure on the surfaces of necrotic neurons, we constructed an in vivo Ca2+ reporter CGaMP5 that was expressed under the control of promoter Pmec-7, a promoter that is specifically active in touch neurons (Furuta et al., 2021). We generated a strain that co-expressed this Ca2+ reporter and the PS reporter MFG-E8::mCherry, and established a time-lapse recording protocol that allowed us to simultaneously monitor the level of Ca2+ in the cytoplasm of two touch neurons (PLML and PLMR) that undergo necrosis and the level of PS on the outer surfaces of these neurons during embryogenesis. This protocol, together with genetic and pharmacological approaches, enabled us to discover that a transient increase in the level of cytoplasmic Ca2+ triggers the exposure of PS on the surfaces of touch neurons during necrosis and that the endoplasmic reticulin (ER) contributes to the PS exposure by releasing Ca2+ to the cytoplasm (Furuta et al., 2021).
Monitoring cytoplasmic Ca2+ and cell surface PS in necrotic cells simultaneously is challenging because long-time imaging of embryos tends to cause photo-damage that impairs proper embryonic development. To overcome this problem, we operated our protocol using the DeltaVision Elite Deconvolution Imaging System from GE Healthcare, Inc., and optimized our experimental settings to minimize photo-damage. Our protocol uses the DeltaVision Elite Deconvolution Imaging System or an equivalent imaging system, the Caenorhabditis elegans strain carrying the Ca2+ and PS reporters, and requires the routine laboratory setup for propagating Caenorhabditis elegans. This protocol can also be adapted to monitor cytoplasmic Ca2+ or PS exposure individually. Our protocol is thus applicable to other Ca2+- and/or PS exposure-related cellular and developmental events. Furthermore, if the Pmec-7 promoter is replaced by other promoters, this protocol can be used to monitor cytosolic Ca2+ in cell types other than the touch neurons.
Materials and Reagents
Reporters
The Ca2+ reporter construct: Pmec-7GCaMP5G
The PS reporter: Pdyn-1mfg-e8::mCherry
Note: The reporter constructs are available upon request (Furuta et al., 2021).
The Caenorhabditis elegans strain that carries both reporters
Strain name: ZH3052
Genotype: enIs92[Pmec-7GCaMP5G , Pdyn-1mfg-e8::mCherry, punc-76(+)]V unc-76(e911)V; mec-4(e1611)X .
Note: This strain is available upon request (Furuta et al., 2021).
Consumables and Reagents
Microscope slides (Premiere, catalog number: 9101)
Coverslips (22 × 22 mm) (Fisher Scientific, catalog number: 12-542B)
DeltaVision immersion oil N = 1.516 (Cytiva, catalog number: 29162940)
Handmade worm pick, which is a platinum wire (Alfa Aesar, catalog number: 10287) mounted on a Pasteur pipette (Fisher Scientific, catalog number: 13-678-20B) (Figure 2)

Figure 2. A handmade worm pickHigh vacuum grease (Fisher Scientific, catalog number: 14-635)
Agarose (Fisher Scientific, catalog number: BP160-500)
KH2PO4 (MilliporeSigma, catalog number: PX1565)
Na2HPO4 (MilliporeSigma, catalog number: 567550)
NaCl (Fisher Scientific, catalog number: S671)
NH4Cl (Fisher Scientific, catalog number: S671-3)
4% agarose solution (see Recipes)
M9 buffer (see Recipes)
Equipment
Stereomicroscope (Nikon, catalog number: SMZ645) or any stereomicroscope from other manufacturers for handling Caenorhabditis elegans.
The DeltaVision Elite Deconvolution Imaging System (GE Healthcare, Inc.), including AP1 F1-Delta Vision microscope (Olympus) equipped with 20×, 63×, and 100× Uplan Apo objectives, excitation/emission filter sets, fluorescent light source, DIC microscopy accessories, motorized stage (X, Y, and Z-axis), and a Coolsnap HQ2 digital camera (Photometrics) (Figure 3). For fluorescent imaging, two sets of fluorescence filters (Chroma Inc.) are used, including the GFP filter (excitation wavelength 475/28 nm; emission wavelength 525/50 nm) and the mCherry filter (excitation wavelength 575/25 nm; emission wavelength 632/60 nm). The DeltaVision microscope is kept in a room where the temperature is maintained at 20°C.

Figure 3. DeltaVision deconvolution microscopeA computer for image processing and analysis
Software
SoftWoRx 5.5 Software (for the deconvolution and processing of images) (GE Healthcare, Inc.)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Furuta, Y. and Zhou, Z. (2021). Simultaneous Monitoring Cytoplasmic Calcium Ion and Cell Surface Phosphatidylserine in the Necrotic Touch Neurons of Caenorhabditis elegans. Bio-protocol 11(20): e4187. DOI: 10.21769/BioProtoc.4187.
分类
神经科学 > 细胞机理 > 胞内信号传导
发育生物学 > 细胞信号传导 > 细胞凋亡
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link