- EN - English
- CN - 中文
Isolation of Primary Cytotrophoblasts From Human Placenta at Term
足月人胎盘原代细胞滋养层细胞的分离
(*contributed equally to this work) 发布: 2021年10月05日第11卷第19期 DOI: 10.21769/BioProtoc.4185 浏览次数: 3447
评审: Giusy TornilloDhiman Sankar PalAnonymous reviewer(s)
Abstract
The placenta is a multifaceted organ, fulfilling critical functions for the fetus and the mother. Therefore, it is a critical regulator of the pregnancy, and its dysfunction leads to diseases, including fetal growth restriction and preeclampsia. Studying the placenta is a difficult task since its existence is transient, and its structure is specific to our species. In vitro differentiation of primary cytotrophoblast isolated from term human placenta has been widely used in the placental research field as it represents a reliable model to study cellular differentiation and function. Direct alternatives include trophoblastic cell lines, explants, and organoids, but this protocol, based on the separation of the cells on a Percoll gradient, presents the advantage of being relatively cheap and easy to perform in every research laboratory. Furthermore, the 2D culture is a flexible method that can be adapted to various experimental conditions (transfection, drug exposure, metabolic study, observations, etc.), allowing mechanistic explorations of cellular processes.
Keywords: Trophoblast (滋养层)Background
Traditionally neglected by scientists, the placenta has long been regarded as a temporary organ with basic nutritional functions. Now, it is recognized as a complex and essential organ for mammalian reproduction, as aberrations in its formation and/or function have immediate consequences on pregnancy outcomes, as well as lifelong impacts on the health of the offspring (Malhotra et al., 2019). At term, the placenta has a discoid shape, with one side facing the amniotic cavity (the chorionic plate) and the other in contact with the endometrium (the decidual plate). Its microstructure is formed of villi that could be compared to multiple trees, which come from the branches of the fetal arteries (Turco and Moffett, 2019). Since these villi are directly in contact with the maternal blood, the human placenta is told hemochorial (Foidart et al., 1992). The interface between the maternal blood and the fetal vessels is called the syncytium. This unique layer constitutes the functional unit of the placenta and comes from the fusion of the mononuclear villous cytotrophoblasts (CTBs) to form multinucleated syncytiotrophoblasts (STBs). This process, known as syncytialization, takes place throughout the pregnancy and is critical for the formation and the functions of the placenta (Costa, 2016). On the other hand, the CTBs can also stay mononuclear but acquire an invasive capacity, especially during the first trimester. In this case, they are called extravillous trophoblasts (EVTs) and are crucial to the adaptations of the maternal arteries to pregnancy (Chang et al., 2018). In both cases, the study of CTBs differentiation represents one of the keys to improve our understanding of placental development and related diseases.
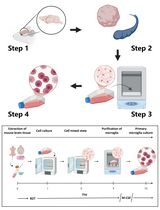
There are no perfect experimental models to investigate the human placenta as the differences between mammals are considerable, even in species with hemochorial placentas (e.g., rodents and non-human primates) (Roberts et al., 2016). Additionally, disorders such as preeclampsia, in which the primary defect is a failure in placentation, are found only in humans (Brosens et al., 2011). For these reasons, fundamental research on placental biology was initially based on ex vivo observations of histologic sections (Burton et al., 1999). In the last decades, various in vitro models based on human material were developed and characterized. However, each in vitro system has its benefits and limitations and should be carefully selected based on the trophoblast subtype, the gestational age of interest, and the type of experiment conducted (Colson et al., 2020b). In this context, primary human trophoblasts isolated from normal placenta at term and cultured in plastic cell culture dishes is an easy, cost-effective, and reproducible method to study specific molecular and genetic regulations in the placenta after the first trimester of gestation. This protocol is based on the method originally described by Kliman (Kliman et al., 1986) but was significantly improved by our team. The main advantages of this technique are its simplicity, its efficiency, and its cost. In addition, the method gives reproducible results as we have been able to characterize many independent cultures of several days (up to 12 days) over the last 10 years (Debieve et al., 2013; Depoix et al., 2013, 2016, 2018 and 2020; Colson et al., 2020a). Furthermore, the 2D culture is a flexible method that can be adapted to various experimental conditions (transfection, drug exposure, metabolic study, observations, etc.), allowing mechanistic explorations of cellular processes.
Limitations of this technique are the need for ethical permission, a good liaison with clinical staff, and the innate variability between samples. However, this latter limitation can be avoided by carefully selecting the placentas (similar gestational age, parity, and maternal condition) and repeated experiments. To overcome these difficulties, many research groups have turned to cell lines, including human embryonic stem cell-derived trophoblast cells (Amita et al., 2013), human trophoblast stem cells (Okae et al., 2018), and choriocarcinoma cell lines. Even if the first two cell lines require further characterization, their use is justified when the study is focused on early placentation as first-trimester trophoblasts are difficult to obtain. However, the use of choriocarcinoma cell lines such as JAR, JEG3, or BeWo to study CTB differentiation and functions poses real methodological difficulties. There are growing concerns about the purity, identity, and behavior of these common cell lines (Abbas et al., 2020). For instance, cAMP must be added to the culture medium to induce BeWo differentiation, while primary CTBs spontaneously fuse in vitro. Finally, genome-wide DNA methylation studies showed significant variability in comparison with primary cells, which is likely to contribute to differential expression profiles (Apps et al., 2011; Novakovic et al., 2011). Therefore, it becomes difficult to draw definitive conclusions when using such cell lines, which highlights the importance of validating findings by using primary trophoblasts.
Another alternative to primary human CTB cell culture is the use of placental explants from human placentas. Their culture is very easy and has the main advantage of keeping the CTBs in their complex tissue environment. It allows the comparison between placentas from normal pregnancies and pathological placentas (preeclampsia or fetal growth restriction) (Orendi et al., 2011). However, studies have shown that this technique impairs trophoblast viability and induces an unnatural oxidative stress (Di Santo et al., 2003; Goncalves et al., 2016). Since then, the use of explants in the study of placental biology must be restricted to comparisons with pregnancy-related diseases and must be limited in time. Finally, 3D cell culture models of human trophoblast cells that closely mimic the villous placenta were recently developed (Haider et al., 2018; Turco et al., 2018). These organoids, composed of a layer of CTBs surrounding a core of STBs, can also be differentiated into EVTs. These models undoubtedly represent a breakthrough in the understanding of placental biology but require specific technical skills and a consequent budget. For these reasons, we are convinced that primary human term CTB cultures present undeniable advantages that still justify their place in the placental field.
Materials and Reagents
Disposable
Percoll (1.130 ± 0.005 g/ml) (GE Healthcare Bio-Sciences AB, catalog number: GE17-0891-01). Keep it sterile and conserve at 4°C
10× Hank’s Buffered Saline Solution w/o Ca, w/o Mg, w/o Phenol red (Gibco, Thermo Fisher Scientific, catalog number: 14175053). Keep it sterile and conserve at 4°C
1× Hank’s Buffered Saline Solution w/o Ca, w/o Mg, w/o Phenol red (Gibco, Thermo Fisher Scientific, catalog number: 14175095). Keep it sterile and conserve at 4°C
Dulbecco's modified Eagle medium (Thermo Fisher Scientific, catalog number: 12440061). Keep it sterile and conserve at 4°C
Iscove’s Modified Dulbecco Medium (Gibco, Thermo Fisher Scientific, catalog number: 12440061). Keep it sterile and conserve at 4°C
Gentamicin 50 mg/ml (Gibco, Thermo Fisher Scientific, catalog number: 15750037). Keep it sterile and conserve at RT
Sterile heat-inactivated Fetal Bovine Serum (Gibco, Thermo Fisher Scientific, catalog number: 10082147). Keep it sterile. Aliquote and store at -20°C
Dulbecco's phosphate-buffered saline (DPBS) (Gibco, Thermo Fisher Scientific, catalog number: 14190359). Keep it sterile and conserve at 4°C
Lyophilized dispase II (Roche Diagnostics, catalog number: 4942078001). Store at 4°C
DNAse I, grade II (Roche Diagnostics, catalog number: 10104159001). Resuspend at 10 mg/ml and store at -20°C
4 L to 8 L NaCl 0.9% (Carl Roth GmbH + Co. KG, catalog number: 9265.2). Keep it sterile and conserve at 4°C
Nunc 200 ml conical centrifuge tubes (Thermo Fisher Scientific, catalog number: 376813)
15 ml centrifuge tubes (Greiner Bio-One International GmbH, catalog number: 188261)
50 ml centrifuge tubes (Greiner Bio-One International GmbH, catalog number: 227261)
5 ml serological pipets (Greiner Bio-One International GmbH, catalog number: 606180)
10 ml serological pipets (Greiner Bio-One International GmbH, catalog number: 607180)
20 ml syringes (Greiner Bio-One International GmbH, catalog number: 474203)
50 ml syringes (Greiner Bio-One International GmbH, catalog number: 475203)
Blunt-end 16 G needles (VWR International, catalog number: POPR7938)
Corning 40 µm disposable cell strainer (Corning, catalog number: 431750)
Plastics for cell culture. Coating with collagen or laminin is not necessary, but better results can be obtained by using pretreated plastics such as the Advanced TC products (Greiner Bio-One International GmbH, catalog number: 665980)
Non-Disposable Sterilized Materials
One dish stainless-steel (Carl Roth GmbH + Co. KG, catalog number: 0376.1)
One pair of big scissors (Carl Roth GmbH + Co. KG, catalog number: YE80.1)
One pair of little scissors (Carl Roth GmbH + Co. KG, catalog number: HEY6.1)
One tweezer straight (Carl Roth GmbH + Co. KG, catalog number: 2802.1)
One big round sieve (Carl Roth GmbH + Co. KG, catalog number: 8101.1)
One little round sieve (Carl Roth GmbH + Co. KG, catalog number: 8096.1)
One funnel (Carl Roth GmbH + Co. KG, catalog number: 0166.1)
One flat glass recipient (Carl Roth GmbH + Co. KG, catalog number: HX16.1)
One 500 m baffled flasks with screw closure (Carl Roth GmbH + Co. KG, catalog number: PK17.1)
One 500 ml baffled flasks, straight neck (Carl Roth GmbH + Co. KG, catalog number: LY96.1)
One 1 L beaker (Carl Roth GmbH + Co. KG, catalog number: X695.1)
One 2 L beaker (Carl Roth GmbH + Co. KG, catalog number: X696.2)
One stainless-steel test sieve, pore size 200 µm (Retsch, catalog number: 60.106.000200)
One stainless-steel test sieve, pore size 100 µm (Retsch, catalog number: 60.106.000100)
One stainless-steel test sieve, pore size 40 µm (Retsch, catalog number: 60.106.000040)
Equipment
Pipet controller
Vacuum machine
Bucket
Refrigerated centrifuge at 4°C with adaptors for 15 ml tubes, 50 ml tubes, and 200 ml centrifugation tubes
Stand with a clamp
Surgical lamp (cold light)
Laminar flow hood
Atmosphere-controlled humidified incubator
Scale
Shaking water bath at 37°C
Fluorescence microscope and camera
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Colson, A., Depoix, C. L., Hubinont, C. and Debiève, F. (2021). Isolation of Primary Cytotrophoblasts From Human Placenta at Term. Bio-protocol 11(19): e4185. DOI: 10.21769/BioProtoc.4185.
分类
发育生物学 > 繁殖
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link