- EN - English
- CN - 中文
Phalloidin Staining of Actin Filaments for Visualization of Muscle Fibers in Caenorhabditis elegans
秀丽隐杆线虫肌动蛋白丝的Phalloidin染色用于肌纤维可视化
发布: 2021年10月05日第11卷第19期 DOI: 10.21769/BioProtoc.4183 浏览次数: 4939
评审: Olli Matilainen Gawain McCollAnonymous reviewer(s)
Abstract
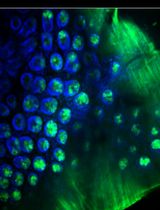
Advances in C. elegans research have allowed scientists to recapitulate different human disorders, from neurodegenerative diseases to muscle dysfunction, in these nematodes. Concomitantly, the interest in visualizing organs affected by these conditions has grown, leading to the establishment of different antibody- and dye-based staining protocols to verify tissue morphology. In particular, the quality of muscle tissue has been largely used in nematodes as a readout for fitness and healthspan. Phalloidin derivatives, which are commonly used to stain actin filaments in cells and tissues, have been implemented in the context of C. elegans research for visualization of muscle fibers. However, the majority of the phalloidin-based protocols depend on fixation steps using harmful compounds, preparation of specific buffers, and large amounts of worms. Herein, we implemented a safer and more flexible experimental procedure to stain actin filaments in C. elegans using phalloidin-based dyes. Lyophilization of the worms followed by their acetone permeabilization allows bypassing the fixation process while also providing the opportunity to suspend the experiment at different steps. Moreover, by using conventional buffers throughout our protocol, we avoid the additional preparation of solutions. Finally, our protocol requires a limited number of worms, making it suitable for slow-growing C. elegans strains. Overall, this protocol provides an efficient, fast, and safer method to stain actin filaments and visualize muscle fibers in C. elegans.
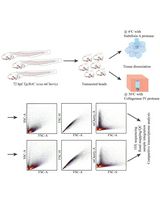
Graphic abstract:

Schematic overview of phalloidin staining in C. elegans for assessing muscle fiber morphology.
Background
In the last decades, the nematode Caenorhabditis elegans has emerged as a powerful model organism and has provided an unprecedented opportunity to expand our knowledge in different fields of research, from developmental biology (Mello et al., 1992; Bowerman et al., 1993; Hubbard and Greenstein, 2000), to neuromuscular degeneration (Braungart et al., 2004; McColl et al., 2012; Sorrentino et al., 2017; Romani et al., 2021) and aging (Kimura et al., 1997; Herndon et al., 2002). One of the main reasons that promoted the usage of C. elegans as a laboratory model is that despite its simplicity, it has defined tissues. In particular, it consists of five main tissues: epidermis, reproductive tissues, digestive system, nervous system, and muscle tissue (Spencer et al., 2011). Among these, muscle tissue morphology and function have been widely used to typify C. elegans fitness and healthspan (Fire et al., 1991; Ryu et al., 2016; Fang et al., 2017; D’Amico et al., 2019; Romani et al., 2021). In recent years, numerous protocols have emerged to facilitate the visualization of C. elegans muscles, taking advantage of the transparent nature of this nematode. Phalloidin is a mushroom-derived molecule that specifically binds filamentous actin (F-actin); hence, phalloidin derivatives tagged with fluorescent molecules are commonly used in microscopy to visualize F-actin in different fields of research (Chazotte, 2010). Body wall muscles of the nematode C. elegans possess obliquely striated myofibrils composed of highly organized filaments of actin, whose disposition is altered in pathological conditions (Gieseler et al., 2018). For this reason, phalloidin-based staining of actin filaments has been successfully implemented in nematodes for the detection of cytoskeleton and muscle structure, with important applications in muscle disease and degeneration (Gieseler et al., 2018). However, phalloidin-based protocols typically rely on tedious fixation steps using harmful compounds, such as PFA, and require the preparation of specific, complicated buffers (Costa et al., 1997; Ono, 2001; Bansal et al., 2015; Geisler et al., 2020). We present here a method for C. elegans research that allows phalloidin staining of actin filaments, bypassing fixation, and using conventional buffers. Moreover, by integrating 4’,6-diamidino-2-phenylindole (DAPI) staining in our protocol, we allow the visualization of muscle cell nuclei, whose morphology can be used to interpret cellular homeostasis. Finally, our protocol requires a limited amount of worms and can be suspended at different steps, ensuring a versatile experimental procedure. Overall, our methodology provides a robust, simple, and safer pipeline for the visualization of muscle fibers and nuclei in C. elegans with applications in multiple fields of biomedical research. We predict that this protocol could be coupled with additional dyes specific for other tissues or cellular components, such as endoplasmic reticulum and mitochondria, allowing an even broader overview of worm physiology and homeostasis.
Materials and Reagents
1.5 ml Eppendorf tube (Eppendorf, catalog number: 11.3817.01)
Glass pipette
Microscope slides (Thermo Scientific, catalog number: 11950657)
Cover slips (VWR, catalog number: 43211.KG)
Plastic dropper (VWR, catalog number: AMP-30)
Aluminum foil
Liquid nitrogen
Acetone (Millipore, catalog number: 1.00014.1011)
Rhodamine Phalloidin (Invitrogen, catalog number: 219920-04-4)
Methanol (Sigma-Aldrich, catalog number: 646377-1L)
BSA (Sigma-Aldrich, catalog number: A7906-100G)
Tween-20 (Sigma-Aldrich, catalog number: P1379)
DAPI (Invitrogen, catalog number: D1306)
Agarose (Promega, catalog number: V3125)
M9 buffer (see Recipes)
Washing buffer (see Recipes)
2 µg/ml DAPI in M9 (see Recipes)
2% (w/v) agarose (see Recipes)
Equipment
Standard equipment for worm culture (see Brenner, 1974)
Centrifuge (Eppendorf, Centrifuge 5424)
SpeedVac Vacuum Concentrator (Labogene, Speed Scan 40)
Laboratory fume hood
Leica SP8 confocal microscope (Stand: Upright Leica DM6 CS; Illumination: Lumencor Sola SM II LED, Laser; Software: LAS-X; Camera: DFC 7000 GT (B/W); Objective: HC PL APO, 63×/1.40 oil, DIC POL0.14)
Software
Fiji (Schindelin et al., 2012)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Romani, M. and Auwerx, J. (2021). Phalloidin Staining of Actin Filaments for Visualization of Muscle Fibers in Caenorhabditis elegans . Bio-protocol 11(19): e4183. DOI: 10.21769/BioProtoc.4183.
分类
发育生物学 > 形态建成 > 细胞结构
发育生物学 > 细胞生长和命运决定 > 肌纤维
细胞生物学 > 组织分析 > 组织成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link