- EN - English
- CN - 中文
Structural and Functional Mapping of Mesenchymal Bodies
间充质体的结构和功能图谱
发布: 2021年10月05日第11卷第19期 DOI: 10.21769/BioProtoc.4177 浏览次数: 4968
评审: Marc-Antoine SaniDinesh MGVishal Nehru
Abstract
The formation of spheroids with mesenchymal stem/stromal cells (MSCs), mesenchymal bodies (MBs), is usually performed using bioreactors or conventional well plates. While these methods promote the formation of a large number of spheroids, they provide limited control over their structure or over the regulation of their environment. It has therefore been hard to elucidate the mechanisms orchestrating the structural organization and the induction of the trophic functions of MBs until now. We have recently demonstrated an integrated droplet-based microfluidic platform for the high-density formation and culture of MBs, as well as for the quantitative characterization of the structural and functional organization of cells within them. The protocol starts with a suspension of a few hundred MSCs encapsulated within microfluidic droplets held in capillary traps. After droplet immobilization, MSCs start clustering and form densely packed spherical aggregates that display a tight size distribution. Quantitative imaging is used to provide a robust demonstration that human MSCs self-organize in a hierarchical manner, by taking advantage of the good fit between the microfluidic chip and conventional microscopy techniques. Moreover, the structural organization within the MBs is found to correlate with the induction of osteo-endocrine functions (i.e., COX-2 and VEGF-A expression). Therefore, the present platform provides a unique method to link the structural organization in MBs to their functional properties.
Graphic abstract:
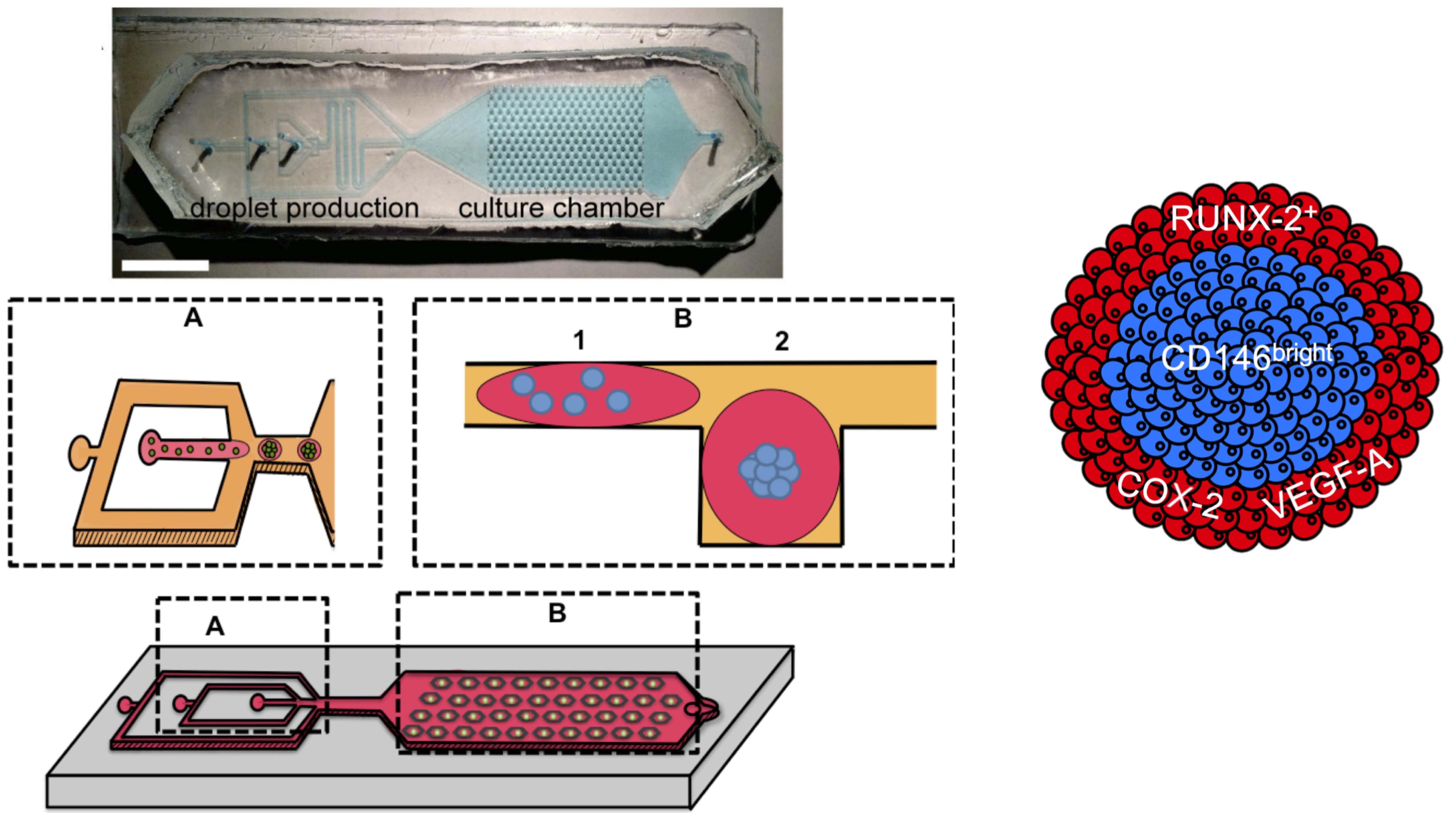
Droplet microfluidic platform for integrated formation, culture, and characterization of mesenchymal bodies (MBs). The device is equipped with a droplet production area (flow focusing) and a culture chamber that enables the culture of 270 MBs in parallel. A layer-by-layer analysis revealed a hierarchical developmental organization within MBs.
Background
Mesenchymal stem/stromal cells (MSCs) comprise a heterogeneous population of mesenchymal progenitors that are capable of differentiation into osteoblastic, chondrogenic, and adipogenic lineages (Dominici et al., 2006). MSCs also bear important trophic functions that regulate immune cell activities, promote angiogenesis, reduce tissue inflammation, and activate tissue-resident progenitors, making this cell type particularly suited for many tissue engineering/regeneration applications (Caplan and Correa, 2011). The formation of spheroids with MSCs (i.e., mesenchymal bodies, MBs) was recently found to enhance their differentiation potential and their secretory activities (Sart et al., 2014). However, it remains poorly understood how heterogeneous hMSCs self-organize in 3D, as well as the mechanisms linking their structural organization to their functional activities (Cesarz and Tamama, 2016).
Several methods have been used for the formation of MBs, including bioreactors and conventional well plates (Sart et al., 2014 and 2016; Sart and Agathos, 2018). However, while these methods enable the formation of a large number of MBs, they provide limited control over single aggregate stimulation or characterization (Sart et al., 2016; Sart and Agathos, 2018). Because MSCs constitute a heterogeneous population, the analysis of single aggregates at single-cell resolution is required to understand the mechanisms by which 3D cultivation induces functional changes on the behavior of a minority of very responsive cells or a global population shift. The macro-scale culture vessels and global population analysis therefore do not allow the MB structure and the cellular functions to be related (Sart et al., 2017; Sart and Agathos, 2018).
Here, we use a microfluidic platform that allows high density and controlled-sized MB formation within nanoliter drops. The microfluidic format enables a robust quantitative demonstration, using quantitative imaging at a single-cell level, that human MSCs self-organize in a hierarchical manner: The most undifferentiated MSCs are located in the core, while partially committed cells are located at the boundaries of the MBs. Moreover, we found that such structural organization correlated with the induction of osteo-endocrine functions. The microfluidic method and protocols developed here can find applications to characterize other types of organoids, to link cell sorting processes to phenotypic commitment, in view of understanding the mechanisms leading to tissue patterning in 3D stem cell cultures. The current method can be applied to any kind of stem cells cultivated in 3D, although the volume of droplets should be adapted to a specific type of stem cells. The data-driven approach developed in this protocol allows us to obtain a robust quantitative characterization of the structural and functional organization within organoids, which would be difficult to obtain using conventional population-based approaches that require the alteration of the cellular microenvironment.
Materials and Reagents
Cell culture reagents
T-175 cm2 flasks (Greiner, Cellstar, catalog number: 660175)
Human mesenchymal stromal cells derived from the Wharton’s jelly of the umbilical cord (hMSCs), purchased from American Type Culture Collection (ATCC) (ref #PCS-500-010)
α-modified Eagle’s medium (α-MEM) (Life Technologies, catalog number: 32561-029)
TrypLETM Express (Life Technologies, catalog number: 12604013)
Phosphate buffer saline (PBS) (Sigma-Aldrich, catalog number: D8662)
Fetal bovine serum (FBS) (Life Technologies, catalog number: 10500-064)
Penicillin- streptomycin (pen-strep) (Life Technologies, catalog number: 10378-016)
Triton X-100 (Sigma-Aldrich, catalog number: X100)
Ultra-low-melting agarose (Sigma-Aldrich, catalog number: A5030)
Paraformaldehyde (PFA), 16% (Alpha Aesar, catalog number: 43368)
Mouse anti-human CD146-Alexa Fluor 647 (clone P1-H12) (BD Biosciences, catalog number: 563619)
Rabbit anti-COX-2 polyclonal antibody (Abcam, catalog number: ab15191)
Rabbit anti-human VEGF-A monoclonal antibody (Abcam, catalog number: ab52917)
Mouse anti-human RUNX-2 monoclonal antibody (Abcam, catalog number: ab76956)
Alexa Fluor 488-conjugated goat anti-mouse IgG2a secondary antibody (Life Technologies, catalog number: A-21131)
Alexa Fluor 594-conjugated goat polyclonal anti-rabbit IgG secondary antibody (Life Technologies, catalog number: A-11012)
DAPI (Sigma-Aldrich, catalog number: 10236276001)
VybrantTM multicolor cell labeling kit (Life Technologies, catalog number: V22889)
hMSC culture medium (see Recipes)
Staining buffer (see Recipes)
CD146 staining solution (see Recipes)
Fixative solution (see Recipes)
Agarose solution (see Recipes)
Permeablization buffer (see Recipes)
Blocking buffer (see Recipes)
Primary antibodies solution (see Recipes)
Secondary antibodies solution (see Recipes)
Materials for microfabrication and microfluidics
Brass plates (5 × 5 cm)
Dry-film photoresists: Eternal Laminar E8020, Eternal Laminar E8013 (Eternal Materials), and Alpho NIT215 (Nichigo-Morton)
K2CO3 (Sigma-Aldrich, catalog number: P5833)
Poly(dimethylsiloxane) (PDMS) (Dow Corning, catalog number: SYLGARD 184)
3MTM NovecTM 1720 Electronic Grade Coating (3M)
3MTM FluorinertTM Electronic Liquid FC-40 (3M)
PEG-di-Krytox (RAN Biotechnologies)
Equipment
Equipment for cell culture
CO2 incubator (Binder, CB 170)
Cell sorter (flow cytometer) (BD Biosciences, FACSAria III)
Equipment for microfabrication and microfluidics
Office laminator (PEAK, pro PS320)
Ultraviolet lamp (Hamamatsu, Lightningcure)
Micromilling machine (Minitech Machinery, CNCMini-Mill/GX)
Plasma cleaner (Harric, PDC-32G)
100 μl and 1 ml glass syringes (SGE, Analytical Science, Gas tight luer lock syringes)
1 ml plastic syringe (Terumo, SS+01T1)
Syringe pumps (neMESYS Low-Pressure Syringe Pump, Cetoni GmbH)
Microscopy
Motorized wide-field microscope (Ti, Eclipse, Nikon), equipped with a CMOS (complementary metal-oxide semiconductor) camera (ORCA-Flash4.0, Hamamatsu), a fluorescence light-emitting diode source (Spectra X, Lumencor), and a 10× objective with a 4-mm working distance (extra-long working distance) and a 0.45 numerical aperture (NA) (Plan Apo λ, Nikon)
Motorized (Ti2, Nikon) confocal spinning disc microscope equipped with lasers (W1, Yokogawa) and the same camera and objective as above
Software
MATLAB (r2016a, MathWorks, Natick, MA)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Sart, S., Tomasi, R. F., Barizien, A., Amselem, G., Cumano, A. and Baroud, C. N. (2021). Structural and Functional Mapping of Mesenchymal Bodies. Bio-protocol 11(19): e4177. DOI: 10.21769/BioProtoc.4177.
- Sart, S., Tomasi, R. F., Barizien, A., Amselem, G., Cumano, A. and Baroud, C. N. (2020). Mapping the structure and biological functions within mesenchymal bodies using microfluidics. Sci Adv 6(10): eaaw7853.
分类
细胞生物学 > 单细胞分析 > 微流体
发育生物学 > 细胞生长和命运决定
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










