- EN - English
- CN - 中文
Zebrafish Embryos as a Predictive Animal Model to Study Nanoparticle Behavior in vivo
斑马鱼胚胎作为研究体内纳米粒子行为的预测动物模型
发布: 2021年10月05日第11卷第19期 DOI: 10.21769/BioProtoc.4173 浏览次数: 4078
评审: Zinan ZhouFrederik J. VerweijMatthew Swire
Abstract
A failure to fully understand the complex in vivo behavior of systemically administered nanomedicines has stymied clinical translation. To bridge this knowledge gap, new in vivo tools are needed to rapidly and accurately assess the nearly infinite array of possible nanoparticle designs. Zebrafish embryos are small, transparent, and easily manipulated animals that allow for whole organism visualization of fluorescently labeled nanoparticles in real time and at cellular resolution using standard microscope setups. Furthermore, key nano-bio interactions present in higher vertebrates are fully conserved in zebrafish embryos, making these animal models a highly predictive and instructive addition to the nanomedicine design pipeline. Here, we present a step-by-step protocol to intravenously administer, image, and analyze nanoparticle behavior in zebrafish embryos and highlight key nano-bio interactions within the embryonic zebrafish corresponding to those commonly found within the mammalian liver. In addition, we outline practical steps required to achieve light-triggered activation of nanoparticles within the transparent embryo.
Graphic abstract:
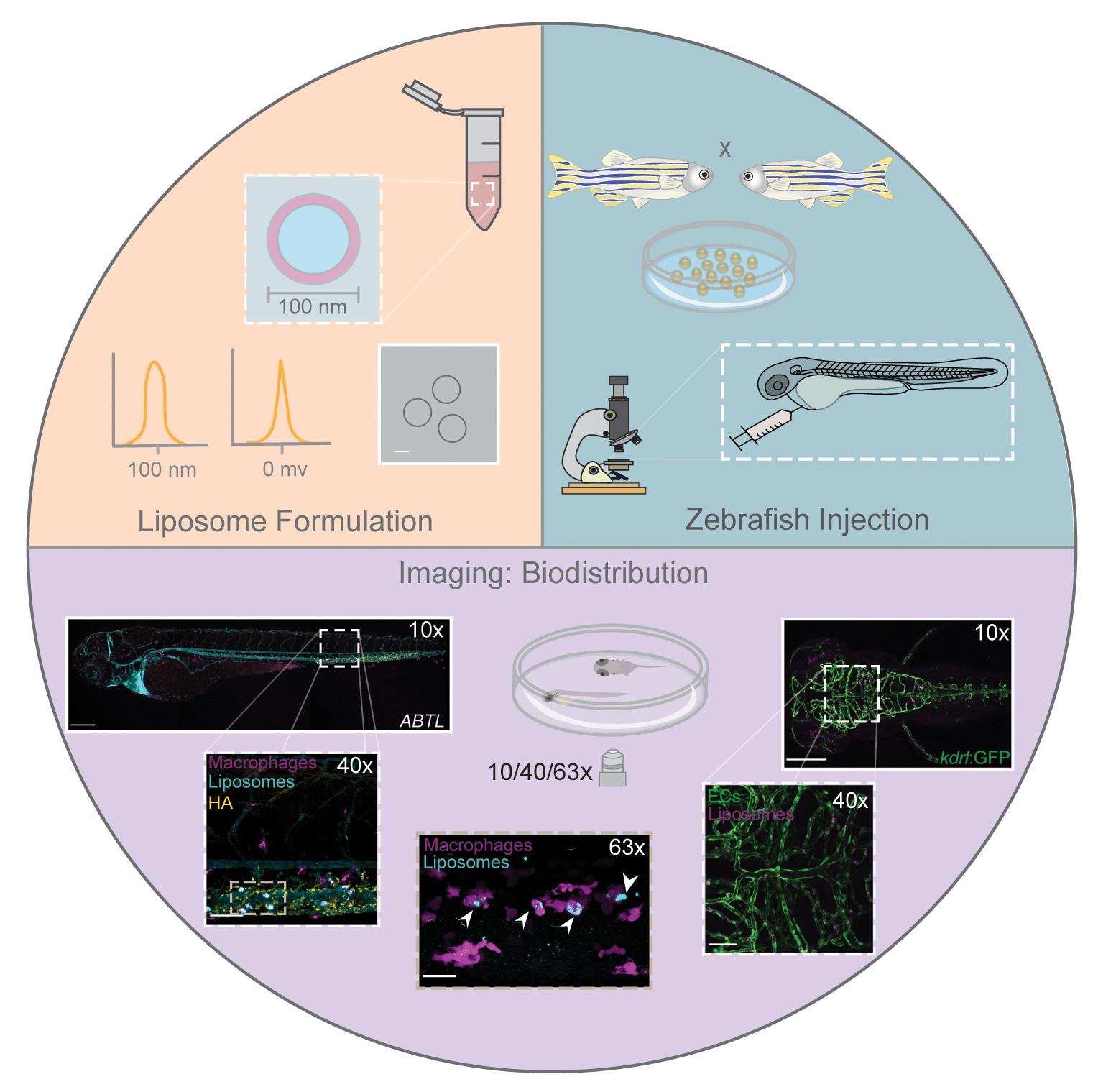
Zebrafish embryos to study nanoparticle behavior in vivo. Formulation, intravenous administration, imaging, and analysis of nanoparticles.
Background
The embryonic zebrafish is a predictive, convenient, and cost-effective animal model to study the complex in vivo behavior of systemically administered nanoparticles and to characterize key nano-bio interactions at a molecular level (Campbell et al., 2018; Sieber et al., 2019a and 2019b; Arias-Alpizar et al., 2020; Hayashi et al., 2020; Arias-Alpizar et al., 2021). In contrast to conventional research animal models (e.g., mice and rats), zebrafish embryos are small (1-3 mm), optically transparent, and readily available in large numbers. These features enable real-time visualization of nanoparticle injected doses using simple fluorescence microscopy setups, at cellular resolution, and over large sample sets. In addition, the extensive range of fluorescent transgenic lines [e.g., mpeg1:GFP (Ellett et al., 2011), macrophages; kdrl:GFP (Jin et al., 2005), endothelial cells], short generational timeframes (approximately 3 months), and the ease of genetic manipulation (e.g., CRISPR/Cas9 gene editing) (Suster et al., 2009; Varshney et al., 2015), facilitates mechanistic understanding of nanoparticle fate in vivo. Crucially, key biological mechanisms underpinning nanoparticle behavior in higher order vertebrates (e.g., rodents and humans) are conserved and functional in zebrafish embryos. In particular, the embryonic zebrafish can accurately predict nanoparticle interactions within the mammalian liver (Campbell et al., 2018; Arias-Alpizar et al., 2021). These interactions account for the (unwanted) clearance of up to 99% of systemically administered nanoparticles (Zhang et al., 2016).
Here, we provide a step-by-step protocol for the intravenous administration, imaging, and analysis of nanoparticles within zebrafish embryos. This protocol describes the use of liposomes but is appropriate for any nanoparticle. In addition, we detail how UV light can be applied to trigger the release of chemical photocages within the embryo in situ and in vivo. As an example, we have recently used UV light to switch the surface charge of liposomes (from neutral to cationic) within the embryonic zebrafish, revealing new insight into the behavior of differently charged nanoparticles in vivo (Arias-Alpizar et al., 2020). In practical terms, a skilled person can inject and mount several hundred zebrafish embryos in a day, and the throughput is generally limited by imaging timeframes. In our experience, confocal imaging (i.e., multi-color whole embryo, 10× objective + 40×/63× ROI), as described below, takes approximately 1 h per embryo. Overall, the embryonic zebrafish is a uniquely powerful addition to the pre-clinical nanomedicine discovery pipeline.
Materials and Reagents
Glass vials, 5 ml (VWR international, catalog number: 548-0555)
Polycarbonate membranes, 400 and 100 nm pore size (Nucleopore Track-Etch membranes, Whatman, catalog numbers: 7065257, 6257028)
Borosilicate glass microneedles with filament, 10 cm (Science Products, Sutter Instruments, catalog number: BF100-78-10)
Microloader 20 μl (Eppendorf, catalog number: 5242956003)
Disposable Petri dishes, 92 × 16 mm with cams (SAERSTEDT, catalog number: 82.1473.001)
Plastic transfer pipettes (SAERSTEDT, catalog number: 86.1171)
Glass bottom dishes (WillCo-dish, catalog number: GWST-5040)
Zebrafish breeding tanks with divider (Tecniplast, Italy)
Adult zebrafish (wildtype (AB/TL) or transgenic line of interest)
Low-melting-point agarose (Sigma-Aldrich, catalog number: A9414)
Instant Ocean sea salt for aquariums (Instant Ocean, catalog number: SS15-10)
Fluorescent nanoparticles (e.g., liposomes as described below; store in the dark)
Lipids (Avanti Polar Lipids, Lipoid GmbH, and/or Sigma-Aldrich)
Chloroform (Sigma-Aldrich, catalog number: 67-66-3)
(Optional) Ethanol (Honeywell, catalog number: 67-63-0)
(Optional) N-Phenylthiourea (PTU) (Sigma-Aldrich, catalog number: P7629; see Recipes; store at room temperature)
Tricaine (ethyl 3-aminobenzoate methanesulfonate) (Sigma-Aldrich, catalog number: A5040) (see Recipes; store at 4°C and in the dark once diluted)
Formulation buffer (e.g., HEPES 10 mM; see Recipes; store at room temperature)
Egg water (see Recipes)
Agarose gel (see Recipes)
Equipment
Stainless steel tip tweezers (IDEAL-TEK, catalog number: 3480641)
Mini-extruder (Avanti Polar Lipids, catalog number: 610000)
Syringes 1000 µl (Avanti Polar Lipids, catalog number: 610017)
Vacuum desiccator (Fisher Scientific, model: Pirex 1594/02D)
Bench-top vortex (Scientific Industries, model: G-560E)
Nanosizer (Malvern Zetasizer Nano ZS)
LED-UV light source (wavelength 370 nm, FWHM = 13.4 nm; H2A1-H375-S, Roithner Lasertechnik)
Stereo microscope (Leica, model: MS5)
Micropipette puller (Sutter Instruments, model: P-97)
Injector (Eppendorf, FemtoJet, catalog number: 524702135) attached to a manual micromanipulator (World Precision Instruments, WPI model M3331R) on a steel base plate (WPI, code 5052) with a magnetic stand (WPI, code M10)
Incubator (Heraeus, model: B15)
Water bath (ELBANTON, Julabo, model: MWB)
Fluorescent stereo microscope (Leica, model: M205 FA-2)
Confocal microscope (Leica Microsystems, model: SP8/SPE)
Fully approved zebrafish facility (see Ethics section below)
Software
Zetasizer Software, version 7.13
Fiji distribution of ImageJ, versions 1.51p and 1.52p (Schindelin et al., 2012; Schneider et al., 2012)
Confocal microscopy data were processed using Leica Software (Leica Application Suite X software, version 3.5.5.19976)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Arias-Alpizar, G., Bussmann, J. and Campbell, F. (2021). Zebrafish Embryos as a Predictive Animal Model to Study Nanoparticle Behavior in vivo. Bio-protocol 11(19): e4173. DOI: 10.21769/BioProtoc.4173.
分类
生物工程 > 生物医学工程
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










