- EN - English
- CN - 中文
Inter-species Transplantation of Blastocysts between Medaka and Zebrafish
青鳉与斑马鱼间囊胚的种间移植
发布: 2021年09月20日第11卷第18期 DOI: 10.21769/BioProtoc.4166 浏览次数: 3740
评审: Giusy TornilloAlberto RissoneAnonymous reviewer(s)
Abstract
Transplantation of blastocysts from a donor to a host blastula constitutes a powerful experimental tool to tackle major developmental biology questions. The technique is widely implemented in diverse biological models including teleost fish, where it is typically used for intra-species blastula transplantations – i.e., labeled blastocysts into a non-labeled host to follow lineages, or mutant blastocysts into a wild-type host to address autonomous vs. non-autonomous roles of a gene of interest. We have recently implemented a protocol to transplant blastocysts between zebrafish (D. rerio) and medaka (O. latipes), two species in which blastocysts show different developmental dynamics and sizes (Fuhrmann et al., 2020). We present here a detailed protocol on how to overcome the early differences in chorion structure, blastula size, and speed of development to achieve trans-species blastocyst transplantation.
Keywords: Blastomere transplantation (胚叶细胞移植)Background
The Chimera is a mythological creature containing parts of different animals – the body of one species, the head of another species, the tail of yet another species, and so on. In genetics, the term is used to define an organism where cells have diverse genetic information – usually a different genetic origin. In the field of developmental biology, chimeras and genetic mosaics have long been used as powerful tools to differentiate cell intrinsic from cell extrinsic processes, as well as to establish lineages (Le Douarin, 1993; Le Douarin and Dupin, 1993; Buckingham and Meilhac, 2011; Kretzschmar and Watt, 2012). The generation of chimeras and mosaics can be done genetically or mechanically, depending on the animal model of choice. For teleost fish, their external development allows the early, mechanical mixing of blastomeres among siblings and/or between a wild type and a mutant embryo. The use of a tracer in donor cells is typically used to identify the foreign cells in the developing host. There are well-established protocols for blastomere transplantation in different species of teleost fish – examples of blastula transplantation in the context of a specific biological question and explanatory videos on the process can be found (Haas and Gilmour, 2006; Rembold et al., 2006; Kemp et al., 2009; Centanin et al., 2011). Each of them takes into account the species-specific morphology of the early embryos and their developmental dynamics. Here, we modify existing protocols and establish a pipeline to generate zebrafish/medaka chimeras (Fuhrmann et al., 2020).
Materials and Reagents
Petri dish, 100 mm diameter (Greiner Bio-One, catalog number: 627102)
12/24 well plate (Corning, catalog numbers: 3512 [12-well]; 3527 [24-well])
Agarose coated dishes (homemade): 10 cm diameter, 6 cm diameter (Greiner Bio-One, catalog numbers: 627102 [10 cm diameter]; 628102 [6 cm diameter]) and 6-well plates (Roth, catalog number: ATCO.01). For the coating, use agarose 1% in ERM (medaka) or E3 (zebrafish). The base of the plastic plate should be covered by agarose; 1-2 mm thick is enough.
Cell saver tips (200 μl) (Biozyme, catalog number: 729051)
Borosilicate glass capillaries, 1.2 mm outer diameter × 0.94 mm internal diameter (Mind that these are different from the injection needles. They should NOT contain an internal filament. HARVARD Apparatus, model: 30-0016 GC120T-10 or GC120T-15)
Razor blade (to cut open the borosilicate needle) (Schreiber Instrumente, catalog number: 11-0240)
For Homemade (Air) transplantation device
Tube connector, Syringe Mouthpiece (Narishige, model: CI-1)
Teflon Tubing (Narishige, model: CT-1)
Handle Probe (WPI, catalog number: 2505)
Needle Holder (2 MM Press Port 1.2 MM, WPI catalog number: MPH412)
Epoxy Potting (Gray, WPI, catalog number: 4886)
1 ml Syringe (BD Plastipak, catalog number: REF300013)
Glass Pasteur pipette (WU Mainz, catalog number: 200760)
Glass Pasteur pipette, opened tip
Glass plates (40 mm diameter; Roth, catalog number: T937.1)
Forceps (Dumont No. 5 or No. 55)
Long tips – to handle medaka embryos after dechorionation (Eppendorf, model: 5242956003, Microloader 20 µl in Racks)
Sandpaper (240/180)
Plastic Pasteur pipette (Biosigma, catalog number: 390512)
Sieve to wash zebrafish embryos
Net to collect medaka females (homemade)
Hook to collect medaka embryos (homemade, see details in Figure 1)
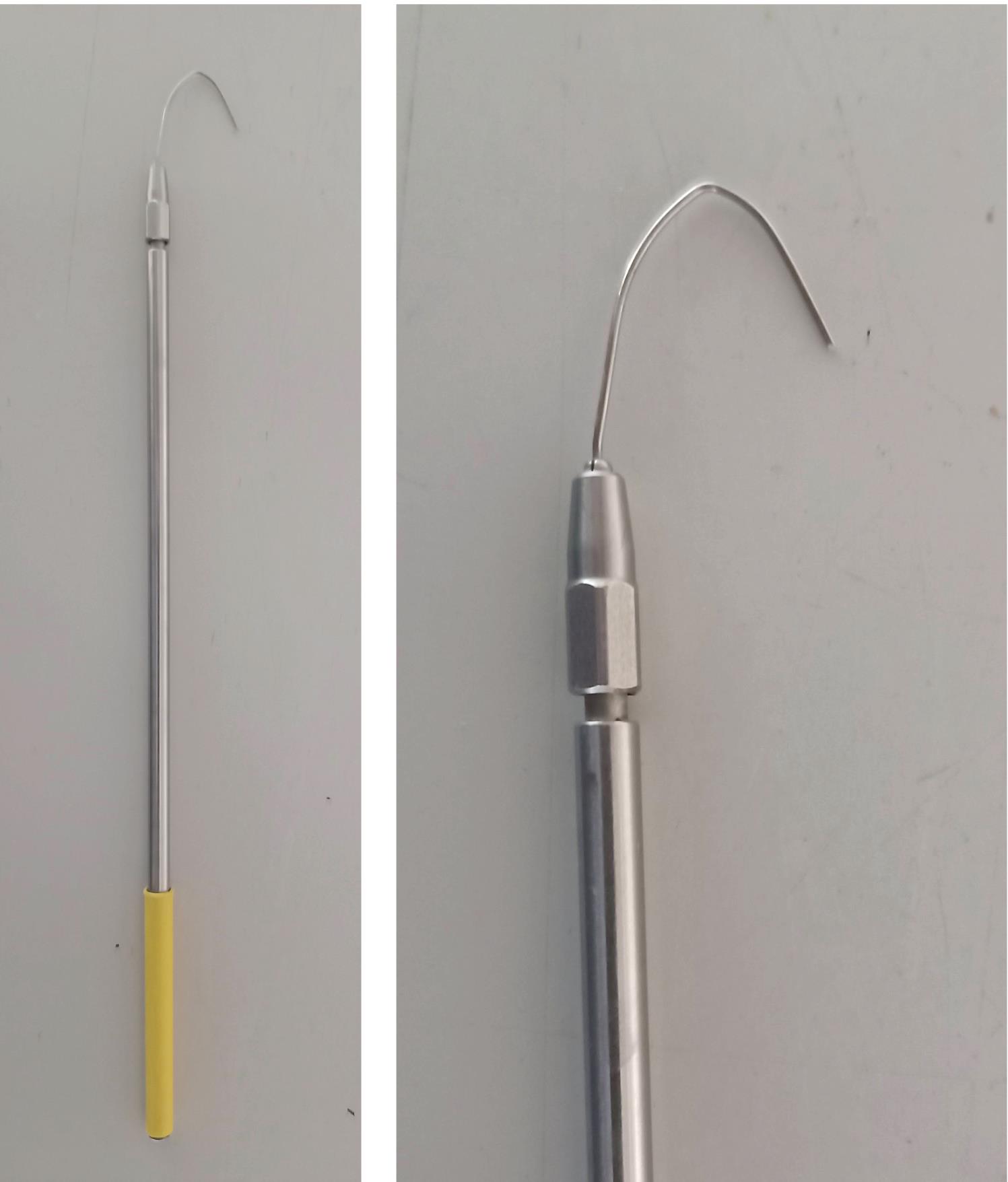
Figure 1. Hook to collect medaka embryos. Inoculation loop holder (Roth, KL97.1), length 21 cm; wire 1.4301: length 2 cm, width 2.5 cm.Glass flasks (100 ml, 500 ml)
100 ml Beakers (Schott, catalog number: 211062402)
Low melting point agarose (Roth, catalog number: 6351.5)
Pronase (Roche, catalog number: 10165921001)
Hatching enzyme (HE) medaka (home-made, can be ordered at NBRP Medaka: https://shigen.nig.ac.jp/medaka/strain/hatchingEnzyme.jsp;jsessionid=8F08DDE7593CDD7D1BFA32E6382E7E0F)
Transplantation mold (we use homemade molds; see Figure 2. Similar commercial molds can be found at: https://www.wpi-europe.com/products/pumps-and-microinjection/oocyte-injection/z-molds.aspx)
Embryo rearing medium for medaka (ERM) (see Recipes)
Zebrafish medium (see Recipes)
20× Tricaine (Sigma-Aldrich, catalog number: A5040-100G) (See Recipes)
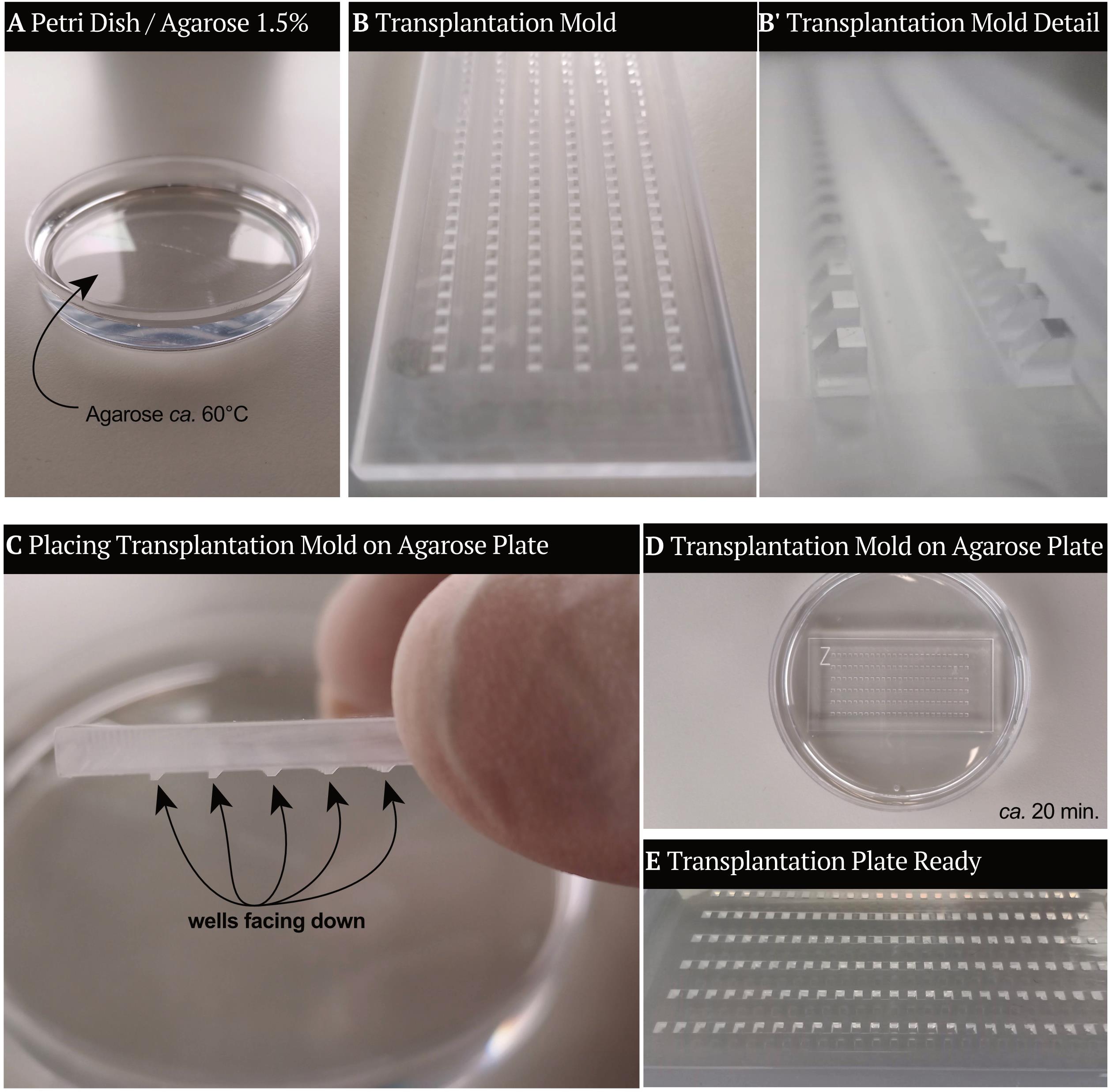
Figure 2. Transplantation molds. To create the transplantation plate, molds are inserted top-down in 1.5% Agarose (see section B1). (A) Petri dish filled with 1.5% agarose in water, ca. 60°C. (B-B’) Transplantation molds with a detailed view of the structures that will form the wells. (C) Transplantation molds are placed in the Petri dish containing agarose, with the wells facing the agarose. (D) Transplantation mold stays in the Petri dish until the agarose has formed a stable gel. (E) When the transplantation mold is removed, the agarose gel contains wells in which the embryos will be placed. The transplantation plate is ready then.
Equipment
Zebrafish housing system (Tecniplast ZEBTEC equipped with 3.5 L tanks)
Zebrafish mating boxes (homemade 5 L boxes; see Figure 3. Alternatively, we have used breeding tanks from Tecniplast – 0.8 or 1.7 L, sloped tank)
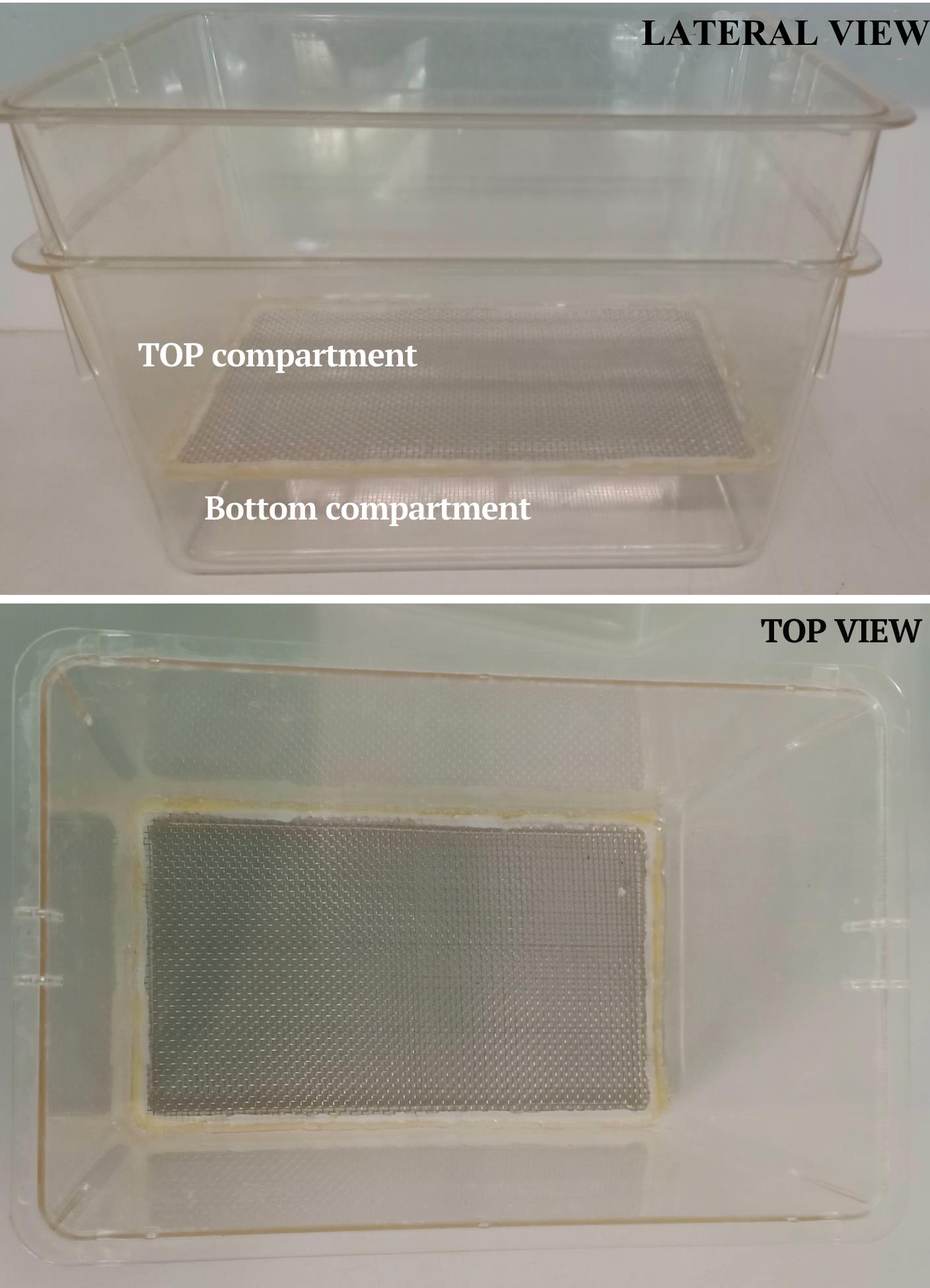
Figure 3. Zebrafish mating box. Lateral view (top) and top view (bottom) of the zebrafish mating box. This setup accommodates up to five couples.Medaka housing system (Tecniplast ZEBTEC equipped with 3.5 L tanks and Müller & Pflege, 4 L tanks)
Incubator, 23°C (no need of CO2 control) (Binder, model: 9020-0339)
Incubator, 28°C (no need of CO2 control) (Rumed, model: 200330160)
Incubator, 32°C (no need of CO2 control) (Binder, model: 9010-0081)
Micropipette puller (we use a horizontal puller, Sutter Instrument CO, model: P-97)
To pull Harvard Apparatus GC120T – 10 capillaries, we use the following program: Heat 505; Pull 25; Vel 250; Time 10)
Transplantation device (a homemade air system; see Figure 4. We have also successfully used the Eppendorf CellTramR Air/Oil system)
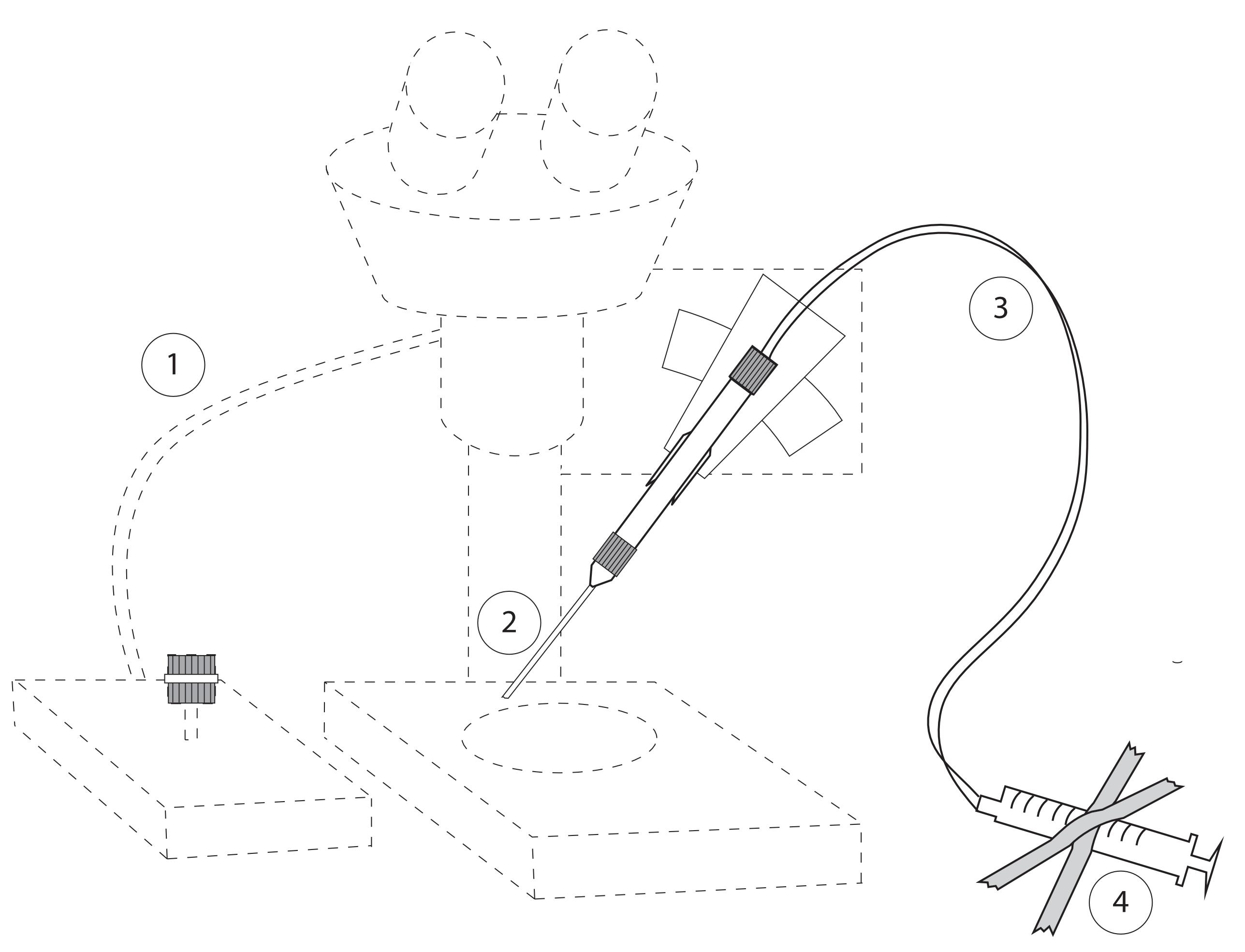
Figure 4. Set up for Transplantations. Main components: (1) Micromanipulator and Stereoscope with light source; (2) transplantation needle inserted into needle holder; (3) tube attached to the needle and a syringe (airtight); (4) syringe (volume 1 ml) attached to desk.Stereoscope with light source (Olympus, model: SZX7)
Micromanipulator (Eppendorf, model: InjectMan NI 2)
Pipette (Gilson 20-200 µl, 200-1,000 µl)
Fluorescence stereomicroscope (Nikon, model: SMZ18, solar light engine from Lumencor)
Microwave
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Fuhrmann, J. F., Onistschenko, J. and Centanin, L. (2021). Inter-species Transplantation of Blastocysts between Medaka and Zebrafish. Bio-protocol 11(18): e4166. DOI: 10.21769/BioProtoc.4166.
分类
发育生物学 > 形态建成 > 器官形成
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link