- EN - English
- CN - 中文
Plasmodium cynomolgi Berok Growth Inhibition Assay by Thiol-reactive Probe Based Flow Cytometric Measurement
硫醇反应探针流式细胞术检测食蟹猴疟原虫生长抑制
发布: 2021年09月05日第11卷第17期 DOI: 10.21769/BioProtoc.4147 浏览次数: 3449
评审: Alexandros AlexandratosYee Ling LauAnonymous reviewer(s)
Abstract
The relapsing malaria species, Plasmodium vivax, is the most widely distributed and difficult-to-treat cause of human malaria. The merozoites of P. vivax preferentially invade ephemeral human CD71+ reticulocytes (nascent reticulocytes), thereby limiting the development of a robust continuous culture in vitro. Fortunately, P. vivax’s sister species, P. cynomolgi Berok, can be cultured continuously, providing the ability to screen novel therapeutics drug and vaccine candidates in a reliable and high-throughput manner. Based on well-established growth inhibition activity (GIA) assays against P. falciparum and P. knowlesi, this protocol adopts the current flow cytometry assay methodology and investigates P. vivax inhibitory antibodies using the P. cynomolgi Berok invasion model based on the thiol-reactivity and DNA abundance of viable parasites in macaque erythrocytes. Established GIA assays screen antibodies at either a single concentration or high/low dose concentrations to provide quick insights for prioritizing potential antibodies capable of specifically interrupting parasite ligand and host receptor binding with minimal concentrations. Hence, this protocol expands on the existing GIA assay by using serially diluted antibodies and generating a dose-response curve to better quantify the inhibitory efficacy amongst selected vaccine candidates.
Keywords: Plasmodium vivax (间日疟原虫)Background
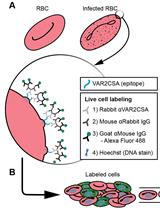
The growth inhibition assay examines the re-invasion and growth of the Plasmodium spp. over two cycles of parasite replication (one cycle is approximately 24 h for P. knowlesi and 48 h for P. vivax, P. cynomolgi, and P. falciparum). After egress, asexual merozoites are released to continuously invade and multiply within host erythrocytes, increasing parasitaemia. During merozoite invasion, a cascade of parasitic proteins interacts with host cell receptors to manipulate host cell entry. In asymptomatic P. vivax patients, monoclonal antibodies recognise a wide range of parasitic ligands such as the duffy binding proteins (DBPs), reticulocyte binding proteins (RBPs), merozoite surface proteins (MSPs), and apical membrane antigen (AMA) (Han et al., 2019; Rawlinson et al., 2019). These antibodies have been shown to be strain-transcendent, confer protective immunity, and reduce merozoite invasion to reticulocytes in vitro short-term culture (Russell et al., 2011). However, the lack of a successfully multiplying continuous culture system of P. vivax reduces the detection signal in the growth inhibition assay (GIA) (Gunalan et al., 2020). To overcome this limitation, past P. vivax studies utilized surrogate species such as P. knowlesi, P. cynomolgi, and P. falciparum to screen inhibitory antibodies (Mitran et al., 2019; Collins et al., 1999; Muh et al., 2020).
The conventional GIA quantifies parasite viability and density using the SYBR green dye and lactate dehydrogenase (LDH) enzyme via flow cytometry and spectrophotometry, respectively (Mohring et al., 2020). Our approach explores thiol reactivity quantification in P. cynomolgi, which reliably assesses P. falciparum parasitaemia in antibody-dependent cellular inhibition assays (Jogdand et al., 2012). The quantification is based on the accumulation and fluorescent covalent biconjugates formed by the thiol-reactive probe carbocyanin, which acts as an indicator for the parasite’s viability in the sarcoplasmic reticulum and mitochondria of eukaryotes (Habicht and Brune 1980; Amaratunga et al., 2014). As the Mitotracker product is available in a wide range of fluorescent dyes and probes, this method also presents an opportunity to investigate other fluorescent channels in flow cytometry. Furthermore, our approach adapts the mean inhibitory concentration approach commonly used in drug assays, allowing researchers to select amongst highly effective inhibitory antibodies with low inhibitory concentrations.
Materials and Reagents
1.5 ml microcentrifuge tube (Axygen®, catalog number: MCT-150-C)
15 ml centrifuge tube (Corning, Costar, catalog number: CLS430052)
50 ml centrifuge tube (Corning, Costar, catalog number: CLS430291)
24-well flat-bottom plates (Corning, Costar, catalog number: CLS3738)
96-well round-bottom plates (Corning, Costar, catalog number: CLS3367)
96-well flat-bottom plates (Corning, Costar, catalog number: CLS3370)
Non-vented T75 flasks (Corning, Costar, catalog number: CLS3814)
3 ml round bottom polystyrene tubes (Becton Dickinson, catalog number: 156758)
Sterile V-channel reservoirs (VWR, catalog number: VWRI613-1174)
Sterile 14 mm Petri dish (VWR, catalog number: VWRI390-1373)
Non-woven filters (Antoshin)
Aluminium foil
Lithium heparin vacuteiners (Becton Dickinson, catalog number: 367880)
Clot activator vacuteiners (Becton Dickinson, catalog number: 367820)
P. cynomolgi Berok erythrocyte culture (Bruce Russell, University of Otago) (Chua et al., 2019)
Macaca fascicularis erythrocytes (Monash Animal Research Platform, Monash University)
M. fascicularis serum (Monash Animal Research Platform, Monash University)
Pre-immune and P. vivax antibodies (Yang Cheng, Jiangnan University) (Shen et al., 2021)
RPMI 1640 medium supplemented with GlutaMAX (Gibco, catalog number: 61870-036)
HEPES (Sigma-Aldrich, catalog number: H4034)
D-glucose (Sigma-Aldrich, catalog number: G7021)
Hypoxanthine (Calbiochem, catalog number: 4010BC)
PBS tablets (Gibco, catalog number: 18912014)
Giemsa (VWR, catalog number: 352603R)
Methanol (Fisher Scientific, catalog number: A412-4)
Hoechst (Sigma-Aldrich, catalog number: 63493)
Mitotracker Deep Red (Thermo Fisher Scientific, FM Molecular Probes, catalog number: 22426)
Complete culture media (see Recipes)
Equipment
Class II Microbiological Safety Cabinet
Microplate orbital shaker
Multichannel pipette (12-Channel pipette, 10-100 μl) (Thermo ScientificTM, catalog number: 4661070N)
Benchtop plate centrifuge (Eppendorf, model: 5804R)
Light microscope with 100× oil immersion lens (NIKON, model: YS100)
Canto II flow cytometer (Becton Dickinson, Canto II)
Tri-mix gas with 5% O2, 5% CO2, and the rest with N2
Hypoxia incubator chamber (Stemcell, catalog number: 27310)
37°C incubator
4°C fridge
-20°C freezer
Software
FACSDiva 6.1.3 software (Becton Dickinson)
Flowjo, LLC (Becton Dickinson, Tree Star Inc, https://www.flowjo.com/contact)
Microsoft Excel (Microsoft, https://www.microsoft.com/en-us/microsoft-365/excel)
Prism 8 (GraphPad, https://www.graphpad.com/scientific-software/prism/)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Ong, J. J. Y., Russell, B. and Han, J. (2021). Plasmodium cynomolgi Berok Growth Inhibition Assay by Thiol-reactive Probe Based Flow Cytometric Measurement. Bio-protocol 11(17): e4147. DOI: 10.21769/BioProtoc.4147.
分类
免疫学 > 抗体分析 > 抗体功能
微生物学 > 微生物细胞生物学 > 细胞分离和培养
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link