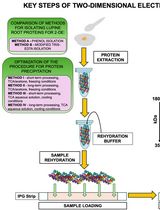
- EN - English
- CN - 中文
Solubilization Method for Isolation of Photosynthetic Mega- and Super-complexes from Conifer Thylakoids
从针叶树类囊体中分离光合巨复合体和超复合体的增溶方法
发布: 2021年09月05日第11卷第17期 DOI: 10.21769/BioProtoc.4144 浏览次数: 4102
评审: Lorenzo FerroniVenkatasalam Shanmugabalaji

相关实验方案
利用SP3珠和稳定同位素质谱技术优化蛋白质合成速率:植物核糖体的案例研究
Dione Gentry-Torfer [...] Federico Martinez-Seidel
2024年05月05日 2852 阅读
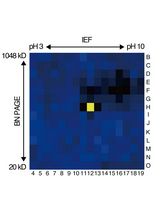
基于活性蛋白质组学和二维聚丙烯酰胺凝胶电泳(2D-PAGE)鉴定拟南芥细胞间隙液中的靶蛋白酶
Sayaka Matsui and Yoshikatsu Matsubayashi
2025年03月05日 1966 阅读
Abstract
Photosynthesis is the main process by which sunlight is harvested and converted into chemical energy and has been a focal point of fundamental research in plant biology for decades. In higher plants, the process takes place in the thylakoid membranes where the two photosystems (PSI and PSII) are located. In the past few decades, the evolution of biophysical and biochemical techniques allowed detailed studies of the thylakoid organization and the interaction between protein complexes and cofactors. These studies have mainly focused on model plants, such as Arabidopsis, pea, spinach, and tobacco, which are grown in climate chambers even though significant differences between indoor and outdoor growth conditions are present. In this manuscript, we present a new mild-solubilization procedure for use with “fragile” samples such as thylakoids from conifers growing outdoors. Here, the solubilization protocol is optimized with two detergents in two species, namely Norway spruce (Picea abies) and Scots pine (Pinus sylvestris). We have optimized the isolation and characterization of PSI and PSII multimeric mega- and super-complexes in a close-to-native condition by Blue-Native gel electrophoresis. Eventually, our protocol will not only help in the characterization of photosynthetic complexes from conifers but also in understanding winter adaptation.
Keywords: Blue-Native gel electrophoresis (凝胶电泳)Background
Being the basis of life on Earth, photosynthesis has become a major research focus from the mid-19th century (Hill, 1937 and 1939; Benson and Calvin, 1950; Benson et al., 1950; Porter, 1950; Huzisige and Ke, 1993). Among several biochemical characterization methods, separation in polyacrylamide gel-based matrix of photosynthetic multi-subunit complexes, such as Photosystem I and II (PSI and PSII), was optimized from previous methods (Schägger and von Jagow, 1991; Kügler et al., 1997; Järvi et al., 2011; Haniewicz et al., 2015; Farci et al., 2017; Rantala et al., 2017). Furthermore, the solubilization approach to extract those complexes evolved over the last decades, moving from “harsh” toward “gentle” procedures by using detergents with lower critical micelle concentration (CMC) and at lower concentrations. The same is true for all procedures aimed at extracting membrane protein complexes for structural and functional analyses. Yet, sample preparation and biochemical characterization remain challenging.
In almost all biochemical studies, the isolation of protein complexes is of fundamental importance for the success of any project. Protocols need to be robust and reproducible to minimize variations between extractions. A crucial factor for successful isolation and characterization is the employment of the best-suited detergent depending on the material used, considering the CMC, incubation time, and the temperature during solubilization but also the mixing procedure. It is an even more delicate task to obtain protocols that keep the isolated membrane protein complexes intact, in a close-to-native state. A protein complex in solution does not necessarily have a native and stable structure; additionally, a detergent useful for extraction can be unsuitable for purification procedures and subsequent studies. Thylakoid membranes are solubilized with different detergents, such as n-Dodecyl-β-D-Maltopyranoside (β-DDM), n-Dodecyl-α-D-Maltopyranoside (α-DDM), and digitonin, at an efficiency depending on the thylakoid region (grana or stoma lamellae) and species (Barera et al., 2012; Haniewicz et al., 2013 and 2015; Albanese et al., 2016; Rantala et al., 2017). Isolated photosynthetic complexes have been fully characterized in Arabidopsis thaliana, Pisum sativum L., and Spinacia oleracea L. (Albanese et al., 2016; Rantala et al., 2017; Wood et al., 2018), and partially characterized in other species, such as Nicotiana tabacum L., Physcomitrella patens, Picea abies L. (Norway spruce), Selaginella martensii, Oryza sativa L., and Zea mays L. (D’Amici et al., 2008; Romanowska et al., 2008; Haniewicz et al., 2013 and 2015; Ferroni et al., 2014; Shen et al., 2017; Iwai et al., 2018; Grebe et al., 2019; Kouřil et al., 2020). Different detergent concentrations, mixtures of two detergents, and multi-step strategies (e.g., differential solubilization) are also used to optimize solubilization (Wientjes et al., 2013; Haniewicz et al., 2015). These approaches gained detailed knowledge on oligomeric states, subunit composition, and dynamics in PSII. To achieve reproducibility, most of these studies were performed on chamber-grown plants, but they do not necessarily yield answers to how photosynthetic regulation takes place under natural conditions. Conversely, studies on tree species (e.g., P. abies) growing in a challenging condition (like a boreal winter) provided a more complex picture of photosynthetic acclimation to environmental stress (Bag et al., 2020; Grebe et al., 2020; Chang et al., 2021). For these species, several differences in composition and abundance in photosynthetic complexes were reported: the presence of triple phosphorylation of the novel LHCB1 variant, needed to achieve winter-adapted chloroplast structures (Bag et al., 2020; Grebe et al., 2020); higher-order mega complexes (Kouřil et al., 2020), which are still a matter of debate in higher plants; and the absence of the PSI-NDH complex, mega-complexes, and the so-called M-LHCII complex (Bassi and Dainese, 1992; Nystedt et al., 2013; Kouřil et al., 2016). The latter is most likely an LHCII assembly containing both Lhcb3 and Lhcb6 that are missing in the Pinaceae family of conifers (Kouřil et al., 2016; Grebe et al., 2019) but present in A. thaliana.
Consequently, optimized biochemical procedures for the isolation and characterization of membrane complexes are needed to pursue such challenging studies, compare different species, and gain meaningful insights on structural/functional differences. In this manuscript, we use digitonin to apply and improve a mild-solubilization procedure for isolating thylakoid complexes from Norway spruce (P. abies) and Scots pine (P. sylvestris). The complexes are characterized by Blue-Native gel electrophoresis (BN-PAGE) and denaturing second dimension gel electrophoresis (2D SDS-PAGE). This newly optimized protocol will enable further characterization of PSI and PSII complexes by improving the extraction procedure in a close-to-native state, which, in these species, cannot be achieved by classic α-/β-DDM solubilization. Finally, the availability of this method opens paths for obtaining an in-depth understanding of the winter adaptation processes in evergreen pine trees.
Materials and Reagents
Consumables
Conical bottom microcentrifuge tubes, 1.5 ml
Conical bottom centrifuge tubes, 50 ml and 15 ml
Pipet tips (P1000, P200, and P10)
Plant material
P. abies and P. sylvestris L. plant materials collected from naturally grown trees in the Umeå University campus (63.8202° N, 20.3054° E)
Reagents
Invitrogen NativePAGE Bis-Tris (3-12% w/v gradient and 1.0 mm thickness, catalog number: BN1001BOX)
Precision Plus Protein Dual Color Standards (SDS-PAGE molecular marker; Bio-Rad, catalog number: 1610374)
SYPRO-ruby staining (ThermoFisher, catalog number: S12001)
APS (Ammonium persulfate, Sigma, catalog number: A3678)
Trizma (Sigma-Aldrich, catalog number: 93362)
Tricine (Sigma-Aldrich, catalog number: T0377)
Sodium Dodecyl Sulphate (SDS) (Sigma-Aldrich, catalog number: 436143)
Serva Coomassie Blue G (SERVA Electrophoresis, catalog number: 17524.02)
Bis-Tris (Sigma-Aldrich, catalog number: 14879)
6-aminocaproic acid (Sigma-Aldrich, catalog number: A7824)
Glycerol (Sigma-Aldrich, catalog number: G5516)
miracloth (Merk Millipore)
Grinding buffer (B1) (see Recipes)
Shock buffer (B2) (see Recipes)
Storage buffer (B3) (see Recipes)
Aqueous acetone solution (80%) (see Recipes)
Washing buffer 1 (WB1) (see Recipes)
Washing buffer 2 (WB2) (see Recipes)
Washing buffer 3 (WB3) (see Recipes)
Solubilizing BTH buffer (SB) (see Recipes)
n-Dodecyl-α-D-Maltopyranoside (α-DDM) (see Recipes)
Digitonin (see Recipes)
100 mM α-DDM working solution (see Recipes)
100 mM digitonin working solution (see Recipes)
Loading buffer (LB) (see Recipes)
Running buffer (RB) (see Recipes)
Soft-staining solution (see Recipes)
Acrylamide solution A (ThermoFischer) (see Recipes)
Acrylamide solution B (ThermoFischer) (see Recipes)
Cathode SDS buffer (CB) (see Recipes)
Anode SDS buffer (AB) (see Recipes)
Equipment
Blender (Multiblender Coline, Clas Ohlson)
Automatic pipettes (P1000, P200, and P10)
Heating block (95°C)
Cooled microcentrifuge (Eppendorf, model: 4530)
Microcentrifuge tube shaker (OrbitTM Digital Microtube and Microplate Shaker)
Hoefer electrophoretic chamber (model: SE260 Mighty Small II)
Bio-Rad electrophoretic chamber (mini)
Bio-Rad mini-PAGE casting apparatus
Power Supply (Bio-Rad Power pack ultra)
pH meter (Bio-Rad)
UV-VIS spectrophotometer (Lumina, scanning range 150-800 nm)
Software
Chemidoc imaging system (Bio-Rad Image Lab version 6)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Bag, P., Schröder, W. P., Jansson, S. and Farci, D. (2021). Solubilization Method for Isolation of Photosynthetic Mega- and Super-complexes from Conifer Thylakoids. Bio-protocol 11(17): e4144. DOI: 10.21769/BioProtoc.4144.
分类
植物科学 > 植物生物化学 > 蛋白质 > 分离和纯化
生物化学 > 蛋白质 > 分离和纯化
生物化学 > 蛋白质 > 电泳
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link