- EN - English
- CN - 中文
Measurement of LRRK2 Kinase Activity by Proximity Ligation Assay
通过邻近连接测定法测量 LRRK2 激酶活性
发布: 2021年09月05日第11卷第17期 DOI: 10.21769/BioProtoc.4140 浏览次数: 3781
评审: Oneil Girish BhalalaPriyanka DuttaAnonymous reviewer(s)

相关实验方案
用于比较人冷冻保存 PBMC 与全血中 JAK/STAT 信号通路的双磷酸化 CyTOF 流程
Ilyssa E. Ramos [...] James M. Cherry
2025年11月20日 2329 阅读
基于 rAAV-α-Syn 与 α-Syn 预成纤维共同构建的帕金森病一体化小鼠模型
Santhosh Kumar Subramanya [...] Poonam Thakur
2025年12月05日 1633 阅读
Abstract
Missense mutations in leucine rich-repeat kinase 2 (LRRK2) cause forms of familial Parkinson’s disease and have been linked to ‘idiopathic’ Parkinson’s disease. Assessment of LRRK2 kinase activity has been very challenging due to its size, complex structure, and relatively low abundance. A standard in the field to assess LRRK2 kinase activity is to measure the level of substrate phosphorylation (pThr73-Rab10) or autophosphorylation of serine 1292 (i.e., phosphoserine 1292; pS1292). The levels of pS1292 have typically been assessed by western blotting, which limits cellular and anatomical resolution. Here, we describe the method for a novel proximity ligation assay (PLA) that can detect endogenous LRRK2 kinase activity (PLA LRRK2) in situ at cellular and subcellular resolutions. PLA is a fluorescence- or chromogen-based assay that can be used to either (1) detect protein-protein interactions or (2) detect and amplify post-translational modifications on proteins. We used PLA for in situ detection and amplification of LRRK2 autophosphorylation levels at serine 1292. Our findings demonstrate that PLA LRRK2 is a highly sensitive and specific assay that can be used for assessing kinase activity in cultured cells and postmortem tissues.
Keywords: Proximity ligation assay (邻近连接测定)Background
Parkinson’s disease (PD) is the second most common neurodegenerative disease (Dorsey and Bloem, 2018), which is caused by both genetic and environmental factors. Approximately 10% of cases are caused by single gene mutations, while 90% are due to generally unknown causes and are termed idiopathic PD (iPD). Missense mutations in leucine rich-repeat kinase 2 (LRRK2) are the most frequent cause of autosomal dominant PD (Paisan-Ruiz et al., 2004; Zimprich et al., 2004). These mutations result in a toxic gain of function related to elevated LRRK2 kinase activity (West et al., 2005; Greggio et al., 2006). The LRRK2 gene locus is also a risk factor for iPD (Simon-Sanchez et al., 2009), and increased kinase activity has been implicated in the pathogenesis of iPD (Fraser et al., 2016; Di Maio et al., 2018).
Assessment of LRRK2 kinase activity has been challenging until recently. Phosphorylation of the LRRK2 substrate Rab10 has been used as an indicator of elevated kinase activity (Steger et al., 2016). There has been growing consensus that phosphoserine 1292 (pS1292) is an effective surrogate for LRRK2 kinase activity, constituting a reliable readout of kinase activity (Sheng et al., 2012). Several LRRK2 mutations (i.e., G2019S and R1441G) that result in elevated kinase activity also display an increase in pS1292 levels (Sheng et al., 2012). Kinase activity is typically measured by immunoblotting for pS1292, which negates anatomical and cellular resolution. LRRK2 is believed to be a relatively low-abundance protein, and detection of pS1292 with traditional immunocytochemistry is difficult due to problems inherent in detecting a fraction of a sparse protein, such as the need for high antibody concentrations that result in nonspecific signals. Signal amplification, an important feature of the proximity ligation assay (PLA), provides higher sensitivity and allows the use of low concentrations of antibodies, which minimizes nonspecific signals. Additionally, the requirement for molecular proximity of the antibody binding sites to generate a signal effectively filters out off-target or nonspecific antibody binding.
We employed the PLA technique (Weibrecht et al., 2010) to amplify the signal of pS1292 (PLA LRRK2) (Figure 1) which, in turn, allows us to visualize kinase active LRRK2 in a cellular context (Di Maio et al., 2018). This approach relies on two antibodies, one that binds to total LRRK2 protein and another antibody from a different species that binds to the pS1292 residue. Next, species-specific oligonucleotide-labeled secondary antibodies (PLA probes) bind to the respective primary antibody (i.e., anti-mouse PLA Probe Minus binds to mouse anti-LRRK2/N241A and anti-rabbit PLA Probe Plus binds to rabbit anti-LRRK2 pS1292). If both primary antibodies are bound to LRRK2 (in close proximity), the PLA probes will be connected and ligated together into circular DNA. This newly ligated circular DNA is used as template for a rolling circle amplification reaction that amplifies the signal several hundred-fold. Then, fluorescently labeled complementary detection oligonucleotides are hybridized to the amplified signal. The resulting fluorescent product can be visualized by confocal microscopy. We validated PLA LRRK2 in CRISPR/Cas9 genome-edited human embryonic kidney 293 (HEK293) cells LRRK2G2019S/G2019S (kinase active) and LRRK2-/- (kinase deficient). We also used PLA to look at LRRK2 kinase activity in rat brain and post-mortem human brain tissues (Di Maio et al., 2018). Importantly, independent research groups have used our PLA LRRK2 assay to measure LRRK2 kinase activity (Fernandez et al., 2019; Obergasteiger et al., 2020; Bucher et al., 2020). Here, we describe in detail our method for how to investigate LRRK2 kinase activity in situ via PLA LRRK2.
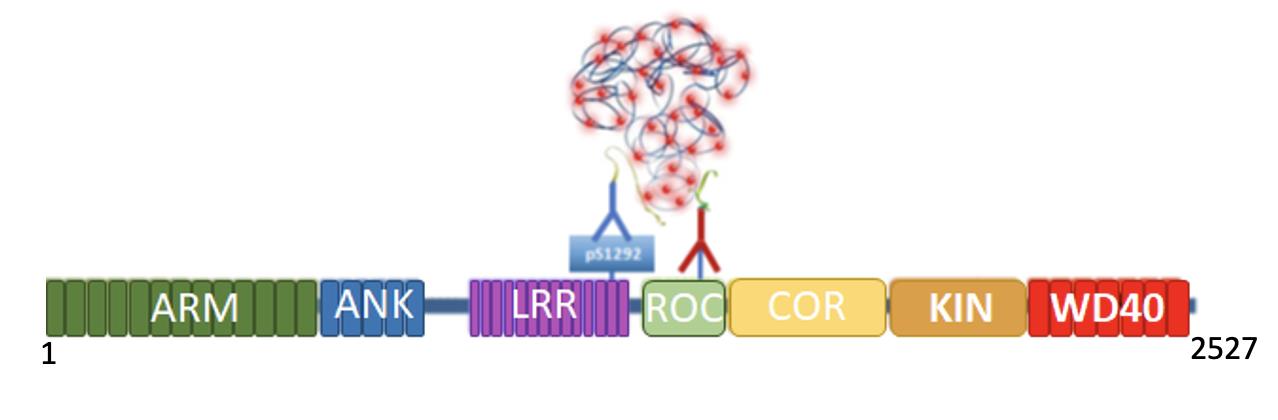
Figure 1. PLA LRRK2 Schematic. In its kinase active form, S1292 undergoes autophosphorylation (pS1292). Therefore, both pS1292 (blue) and total LRRK2 (red) antibodies will bind to LRRK2. The species-specific PLA probes coupled with oligonucleotides recognize a primary antibody (pS1292 or total LRRK2). As both pS1292 and total LRRK2 antibodies are bound to the LRRK2 protein and are in close proximity, the PLA probes are connected and ligated together. This is amplified by a rolling circle amplification reaction, and fluorescent detection oligonucleotides (red dots) are hybridized to this amplified product. The fluorescent signal is visualized by confocal microscopy. ARM, armadillo; ANK, ankyrin; LRR, leucine-rich repeat; ROC, ras of complex; COR, c-terminal of ROC; KIN, kinase.
Materials and Reagents
Fisherbrand Microscope Cover Glass, 12 mm (Fisher Scientific, catalog number: 12-545-80)
Fisherbrand Microscope Cover Glass 22 × 60-1 (Fisher Scientific, catalog number: 205450J)
24-well cell culture plate tissue culture treated (Costar, catalog number: 3526)
Hydrophobic pen
Fisherbrand Superfrost Plus Microscope Slides (Fisher Scientific, catalog number: 22-037-246)
Chamber slides (Tek Chamber Slide w/ Cover glass slide sterile; Thermo Fisher, catalog number: 178599)
Poly-D-lysine hydrobromide (Sigma-Aldrich, catalog number: P6407)
Normal Donkey Serum (NDS) (Jackson Immuno, catalog number: 017-000-121)
Thermo Scientific Nalgene Laptop Cooler (-20°C) (Fisher Scientific, catalog number: 5115-0012)
Triton X-100 (Fisher Scientific, catalog number: BP151-100)
Rabbit anti-LRRK2 phospho-S1292 [MJFR-19-79] (Abcam, catalog number: Ab203181)
Mouse anti-LRRK2/Dardin Clone N241A/34 (UC Davis [Antibodies Inc.], catalog number: 75-253)
Anti-Mouse Minus (Sigma-Aldrich, catalog number: DUO92004-100RXN)
Anti-Rabbit Plus (Sigma-Aldrich, catalog number: DUO92002-100RXN)
Detection Reagents Orange (Sigma-Aldrich, catalog number: DUO92007-100RXN)
Duolink Wash Buffer A and Wash Buffer B (Sigma-Aldrich, catalog number: DUO82049-4L)
16% Paraformaldehyde (10 ml) (Fisher Scientific, catalog number: 50-980-487)
Polyvinyl alcohol (PVA) (Sigma-Aldrich, catalog number: P8136)
Glycerol (Sigma-Aldrich, catalog number: G9012)
Sodium Azide, crystalline (Fisher Scientific, catalog number: S277-100)
Sudan Black B (Sigma-Aldrich, catalog number: 199664)
Histoclear (manufactured by National Diagnostics) (Fisher Scientific, catalog number: HS200)
Sheep anti-tyrosine hydroxylase (Millipore, catalog number: Ab1542)
NDS (see Recipes)
1× PBS (see Recipes)
Wash Buffer A and Washer Buffer B (see Recipes)
Blocking Solution for cells (see Recipes)
Blocking Solution for rat brain tissue (see Recipes)
Blocking Solution for human brain tissue (see Recipes)
Gelvatol Mounting Media (see Recipes)
Equipment
Pipettes (ranging from 1 µl to 1,000 µl)
37°C, 5% CO2 cell culture incubator
Orbital shaker at room temperature (for fixation, permeabilization, and washes)
Orbital shaker at 4°C (for primary antibody incubation overnight)
Incu-shakerTM Mini with nonslip rubber mat (Benchmark Scientific, catalog number: H1001-M)
Note: You can use any other humified incubator at 37°C.
Olympus BX61 confocal microscope (or any confocal microscope)
Software
Olympus Fluoview 1000 Software was used in this analysis; however, any other software that is suitable for image analysis can also be used.
Nikon elements, ImageJ
GraphPad PRISM
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Keeney, M. T., Hoffman, E. K., Greenamyre, J. T. and Di Maio, R. (2021). Measurement of LRRK2 Kinase Activity by Proximity Ligation Assay. Bio-protocol 11(17): e4140. DOI: 10.21769/BioProtoc.4140.
- Di Maio, R., Hoffman, E. K., Rocha, E. M., Keeney, M. T., Sanders, L. H., De Miranda, B. R., Zharikov, A., Van Laar, A., Stepan, A. F., Lanz, T. A., Kofler, J. K., Burton, E. A., Alessi, D. R., Hastings, T. G. and Greenamyre, J. T. (2018). LRRK2 activation in idiopathic Parkinson's disease. Sci Transl Med 10(451).
分类
神经科学 > 神经系统疾病 > 帕金森氏症
神经科学 > 细胞机理
分子生物学 > 蛋白质 > 磷酸化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link