- EN - English
- CN - 中文
Development and Quantitation of Pseudomonas aeruginosa Biofilms after in vitro Cultivation in Flow-reactors
流动反应器体外培养铜绿假单胞菌生物膜的研制与定量
发布: 2021年08月20日第11卷第16期 DOI: 10.21769/BioProtoc.4126 浏览次数: 3677
评审: Alexandros AlexandratosJan-Ulrik DahlAnonymous reviewer(s)
Abstract
Characterization of biofilm formation and metabolic activities is critical to investigating biofilm interactions with environmental factors and illustrating biofilm regulatory mechanisms. An appropriate in vitro model that mimics biofilm in vivo habitats therefore demands accurate quantitation and investigation of biofilm-associated activities. Current methodologies commonly involve static biofilm setups (such as biofilm assays in microplates, bead biofilms, or biofilms on glass-slides) and fluidic flow biofilm systems (such as drip-flow biofilm reactors, 3-channel biofilm reactors, or tubing biofilm reactors). Continuous flow systems take into consideration the contribution of hydrodynamic shear forces, nutrient supply, and physical transport of dispersed cells, which define the habitat for biofilm development in most natural and engineered systems. This protocol describes the assembly of 3 flow-system setups to cultivate Pseudomonas aeruginosa PAO1 and Shewanella oneidensis MR-1 model biofilms, including the respective quantitation and observation approaches. The standardized flow systems promise productive and reproducible biofilm experimental results, which can be further modified according to specific research projects.
Keywords: Biofilm characterization (生物膜的特性)Background
Biofilm is the most prevalent growth mode of microbial organisms in nature, industry, and clinical habitats; it is commonly recognized as bacterial communities embedded in the self-generated matrix of extracellular polymeric substances (EPS) (Flemming et al., 2016). The spatial organization of a biofilm aggregate is highly heterogeneous and has diverse metabolic activities, rendering the biofilm robust and tolerant to numerous types of environmental stress (Van Dyck et al., 2021). Hence, biofilms are promising in industry to ferment for nutrient conversion, degrade hazardous compounds for bioremediation, and generate electricity in microbial fuel cells (Coenye and Nelis, 2010). Conversely, biofilms also raise public concerns such as biofouling and biofilm-associated infections. Biofilms are the leading cause of chronic wound infections and infections on biomedical devices, such as cardiac valves, tracheal tubes, and catheters (Del Pozo et al., 2018). An improved understanding and characterization of biofilm formation, dispersion, and activities will shed light on biofilm control strategies.
A typical biofilm life cycle usually involves 5 stages: initial reversible attachment of bacteria, stable and irreversible attachment, maturation, dispersion, and dispersed free-living bacteria (Martin et al., 2021). The biofilm communities show active social behaviors and interaction with environmental factors, which in return regulate the cellular metabolism of biofilms. Numerous methodologies and devices have been documented for the investigation of biofilm morphology and development in vitro, commonly categorized as static biofilm assays and continuous-flow biofilm systems. The static biofilm assays - such as those in microplates and on air-liquid interface coverslips or colony biofilm assays and Kadouri drip-fed biofilm assays - are prevalently applied to the screening of early events in biofilm formation (Merritt et al., 2005). The static biofilm assays are high-throughput and easily executed with common laboratory equipment; however, nutrient supply in static biofilm assays is limited with respect to developing mature biofilm communities that mimic nature.
Given the fact that biofilms are frequently observed under fluidic flow conditions in nature and engineered systems, nutrient availability, physical transport, and hydrodynamic shear forces significantly impact biofilm formation and metabolism (Mattei et al., 2018). To address the question of how biofilm responds to environmental stresses and communicating signals, it is important to create a standardized and reproducible protocol for cultivating in vitro biofilms in flow systems mimicking the in vivo fluid habitats (Cowle et al., 2020). The development of flow biofilm reactors has been advanced by emerging microfluidics manufacturers and designs, with the most common flow systems including drip-flow systems (Gonzalez et al., 2014), tubular reactors (Winn et al., 2014), planar flow-cell (Zhang et al., 2011), and 3-channel flow-cell (Sternberg and Tolker-Nielsen, 2006; Pamp et al., 2009). Further modifications, such as segmented flow cells (Karande et al., 2014), gradient-generator flow cells (Zhang et al., 2019), and flow-velocity microfluidic flow cells (Liu et al., 2019), are specifically modified and designed to meet specific research purposes.
In this protocol, we addressed three commonly applied flow-system setups for different research purposes with Pseudomonas aeruginosa PAO1 and Shewanella oneidensis MR-1 as model organisms. The tubing biofilm reactor mimics the biofilm habitats in pipelines, tracheal tubes, catheters, etc., allowing biofilm harvest with adequate biomass. The 3-channel flow-cell system is designed for the non-invasive spatiotemporal observation of biofilm morphologies with a continuous and steady nutrient supply. The recycling biofilm reactor enables the study of biofilm metabolic responses toward its bioremediation metabolites and/or environmental chemicals (Sternberg and Tolker-Nielsen, 2006; Weiss Nielsen et al., 2011; Pamp et al., 2019). Overall, the standardized flow systems promise productive and reproducible biofilm observation and quantitation, which can be further modified according to the specific research project.
Materials and Reagents
Silicon tube (I.D=1 mm, O.D=3 mm, Runze, China)
Silicon tube (I.D=3.2 mm, O.D=6.4 mm, Runze, China)
Peristaltic pump tube (I.D=1.02 mm, O.D=2.62 mm, Pharmed BPT, Sain-Gobain)
Straight connector (Runze, catalog number: DI-016)
Straight connector (Runze, catalog number: DI-032)
Luer connector (Runze, catalog numbers: RH-G016; RH-M016)
Needle (Gauge: 26G, Hypodermic; BD, catalog number: 305111)
Syringe (1 ml, BD, Luer-Lock, catalog number: 309628)
Silicone glue (Advanced Silicone 2, GE sealant)
Parafilm (BEMIS, catalog number: PM996)
Metal foil (Maryya, , catalog number: HC081260)
10 μl Inoculation loop (Sangon Biotech, catalog number: F619312)
15 ml Centrifuge tube (Sangon Biotech, catalog number: F602888)
Coverslips (Sangon Biotech, catalog number: F518117)
Microplates (ThermoFisher, NuncTM, catalog number: 168055)
0.22 μm Syringe filter (Sangon Biotech, catalog number: F513163)
2 ml Microtubes (Maxyclear Snapclock, Axygen, US)
LB broth (Sangon Biotech, catalog number: A507002)
Calcium chloride (Sangon Biotech, catalog number: A501330)
Sodium chloride (Sangon Biotech, catalog number: A100241)
QIAamp DNA Micro Kit (QIAGEN, catalog number: 56304)
RNeasy Mini Kit (QIAGEN, catalog number: 74104)
Equipment
Peristaltic pump (200 series 16-channel pump, Watson Marlow)
Microplate reader (Spark®, Tecan, Switzerland)
Flowcytometer (CytoFLEX, Beckman, USA)
Probe sonicator (SONICS, model: VCX750)
Centrifuge (Eppendorf, model: 5418R)
Biosafety Cabinet, BSC (MSC-AdvantageTM II, ThermoFisher, USA)
Autoclave (Zealway, model: GR-60DA)
Confocal Laser Scanning Microscope, CLSM (Zeiss, model: LSM900)
Software
Imaris (Bitplane, Oxford Instrument)
ImageJ (https://imagej.net/)
Comstat 2 (http://www.comstat.dk/)
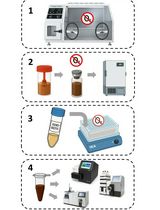
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Zhang, Y., Zhao, J., Cheng, H., Wang, J., Yang, L. and Liang, H. (2021). Development and Quantitation of Pseudomonas aeruginosa Biofilms after in vitro Cultivation in Flow-reactors. Bio-protocol 11(16): e4126. DOI: 10.21769/BioProtoc.4126.
分类
微生物学 > 微生物生物膜 > 群体感应因子
微生物学 > 微生物遗传学 > 基因表达
生物科学 > 微生物学 > 微生物群落 > 微生物组学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link