- EN - English
- CN - 中文
A Genetically Engineered Mouse Model of Venous Anomaly and Retinal Angioma-like Vascular Malformation
静脉畸形和视网膜血管瘤样血管畸形的基因工程小鼠模型
(*contributed equally to this work) 发布: 2021年08月05日第11卷第15期 DOI: 10.21769/BioProtoc.4117 浏览次数: 4141
评审: Pilar Villacampa AlcubierreWilliam C. W. ChenAna Angulo-Urarte
Abstract
Characterization of key regulators in vein development will advance our understanding of mechanisms underlying venous anomalies and provide therapeutic targets for the treatment of vascular malformations. Here, we provide a detailed protocol for the generation of genetically engineered mouse models targeting the Tek gene for the analysis of vein formation and vein-associated vascular diseases at the embryonic and postnatal stages. It includes steps involved in the whole-mount processing of mouse skin, mesentery, and retina for the examination of vascular malformation during embryonic and postnatal development.
Keywords: TIE2 (TIE2)Background
During embryogenesis in mammals, the blood vascular system is one of the first organs to develop from mesoderm-derived hemangioblasts. The developmental process includes the initial fusion of blood islands to form the primitive vascular plexus, followed by vascular specification to form a network comprised of arteries, veins, and capillaries. Characterization of the mechanisms underlying arteriovenous specification will advance our understanding of venous anomalies and provide therapeutic targets for the treatment of vascular diseases. Among the characterized regulators and pathways, the VEGF-A/VEGFR-2 pathway mediates the activation of RAF1 and ERK1/2 kinases to induce the expression of genes required for arterial development (Lanahan et al., 2013; Deng et al., 2013), including Delta-like 4 (Dll4)-mediated activation of NOTCH signaling (Lawson et al., 2001; Duarte et al., 2004; Wythe et al., 2013). Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII, also known as Nr2f2) is a key regulator of venous identity via the inhibition of NOTCH-mediated signals (You et al., 2005). Akt activation inhibits Raf1-ERK1/2 signaling in endothelial cells (ECs) to favor venous specification (Ren et al., 2010); however, the upstream regulators of vein development are poorly characterized. TIE2 is a receptor tyrosine kinase that mediates angiopoietin signaling for EC survival and vascular remodeling and integrity (Augustin et al., 2009). Patients with venous malformations possess Tie2 missense point mutations (Vikkula et al., 1996). TIE2 deficiency leads to embryonic lethality (Dumont et al., 1994; Sato et al., 1995). We have recently demonstrated that TIE2 is essential for the specification of venous EC identity via the Akt-mediated regulation of COUP-TFII protein stability (Chu et al., 2016).
Here, we aim to describe a detailed protocol for analyzing the process of vein development and vein-associated vascular diseases in skin, mesentery, and retina, employing a genetically modified mouse model targeting the Tek gene (Chu et al., 2016).
Materials and Reagents
Plastic pipette (disposable, 5 ml, cut the tip for tissue manipulation)
Needle (ENTO SPHINX s.r.o., 03.15-Minutens white, 03.20-Minutens white)
Insulin syringe (BD, Ultra-Fine®, catalog number: 328421)
Coverslips
4% paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: 158127)
Skimmed milk powder (Valio)
Triton X-100 (Sigma-Aldrich, catalog number: V900502-100ML)
Glycerol (Sigma-Aldrich, catalog number: G5516-500ml)
Rat anti-mouse PECAM-1 (BD Pharmingen, catalog number: 553370)
Goat anti-mouse/rat TIE2 (R&D, catalog number: AF762)
Goat anti-mouse EphB4 (R&D, catalog number: AF446)
Cy3-conjugated anti-mouse αSMA (Sigma-Aldrich, catalog number: C6198)
Alexa Fluor 488-conjugated secondary antibody (Invitrogen, catalog number: A-21208)
Cy3-conjugated secondary antibody (Jackson, catalog number: 705-165-147)
Cy5-conjugated secondary antibody (Jackson, catalog number: 705-175-147)
Tamoxifen (Sigma-Aldrich, catalog number: T5648-5G)
Silicone rubber (SYLGARDTM 184 Silicone Elastomer Kit)
Phosphate-buffered saline (PBS), pH 7.4 (see Recipes)
PBS-TX (see Recipes)
50% glycerol (see Recipes)
Equipment
Confocal laser scanning microscope (Olympus, model: FluoView)
Fluorescence stereomicroscope (Olympus, model: SZX16)
Dissecting scissors (Medical scissors; 66 Vision-Tech Co., catalog number: 54002, straight tip, 100 mm; Vannas capsulotomy scissors: Suzhou Mingren Medical Apparatus and Instruments Co., catalog number: MR-S302A, pointed tips, 16 mm blades)
Stereomicroscope (Motic, catalog number: SMZ168 Series, magnification range: 7.5-50×)
Micro-forceps (Shanghai Medical Instruments Group Ltd., catalog number: WA3040, 14 cm straight, head width 0.3 mm)
Software
FV-ASW Viewer 3.0 or 4.2a
Procedure
Generation of Tek-targeted mouse models for vein analysis in skin and mesentery at the embryonic stage. This protocol is based on the paper by Chu et al. (2016).
Prepare mouse embryos. Place one male mouse (Tek+/-; UBC-CreERT2, ≥ 2 months old) and two female mice (TekFlox/Flox, ≥ 2 months old) into a cage for mating in the late afternoon around 18:00. Check females for vaginal plugs in the early morning of the following day (around 8:00). If the female mouse is pregnant, the embryonic stages are estimated considering midday of the day on which the vaginal plug is present as embryonic day 0.5 (E0.5).
Induce gene deletion. Use sunflower seed oil (COFCO Fortune) as the diluent for the preparation of tamoxifen (tamoxifen free base, 10 mg/ml). Perform intraperitoneal injection of tamoxifen solution (100 μl, 10 mg/ml) from E12.5 to E14.5 (Figure 1A).
Euthanize the pregnant female by cervical dislocation at E17.5 (embryonic day 17.5) and place the mouse on its back on a dissecting board. Use pins to attach the feet of the mouse to the board, and adequately soak the fur with 75% ethanol.
Make a cut in the skin and the abdominal wall with scissors to expose the abdominal cavity and dissect the uterus with scissors.
Separate embryos in ice-cold PBS by removing the uterine muscle layers, pulling the extra-embryonic membranes off with fine forceps, and removing the placenta under a dissecting microscope.
Cut the tip off the tail of the embryos for genotyping.
Peel back the skin with ophthalmic scissors and fix the skin tissues to the 6-well plate containing silicone rubber using fine needles (Figure 1B).
Open the abdomen of the embryo. Cut a fragment of the small intestine long enough to form a ring to keep the mesentery intact for vascular visualization. Fix the intestine to the 6-well plate containing silicone rubber with fine needles (Figure 2A).
Fix the tissues with 4% paraformaldehyde (PFA) at 4°C or on ice for 2 h. Wash 3 times with PBS solution for 5 min each.
Block the tissues with PBS-TX solution containing 3% skimmed milk powder at 4°C for 6-8 h with gentle agitation on an orbital shaker.
Remove the blocking solution and incubate the tissues with primary antibodies (PECAM-1, αSMA, TIE2; dilution according to the manufacturer’s recommendation) in blocking solution (total volume: 1 ml) with gentle agitation on an orbital shaker overnight at 4°C.
Wash the tissues 5 times with PBS-TX for 20 min each.
Incubate the tissues with secondary antibodies (Alexa Fluor 488, Cy5-conjugated secondary antibodies) in PBS-TX solution (dilution 1:500) with gentle agitation on an orbital shaker overnight at 4°C.
Cut off the excess tissues. Transfer the trimmed skin onto a glass slide, add a drop of 50% glycerol, place a coverslip and seal with nail polish at the edges. For the analysis of intestines, transfer the trimmed intestine directly onto a coverslip and keep the mesentery fully stretched under a dissecting microscope. Avoid drying of tissues by adding drops of 50% glycerol.
Analyze blood vessels under a confocal laser scanning microscope (Olympus FluoView) and acquire and process images with the FV-ASW Viewer 3.0 /4.2a software (Figures 1C and Figure 2B).
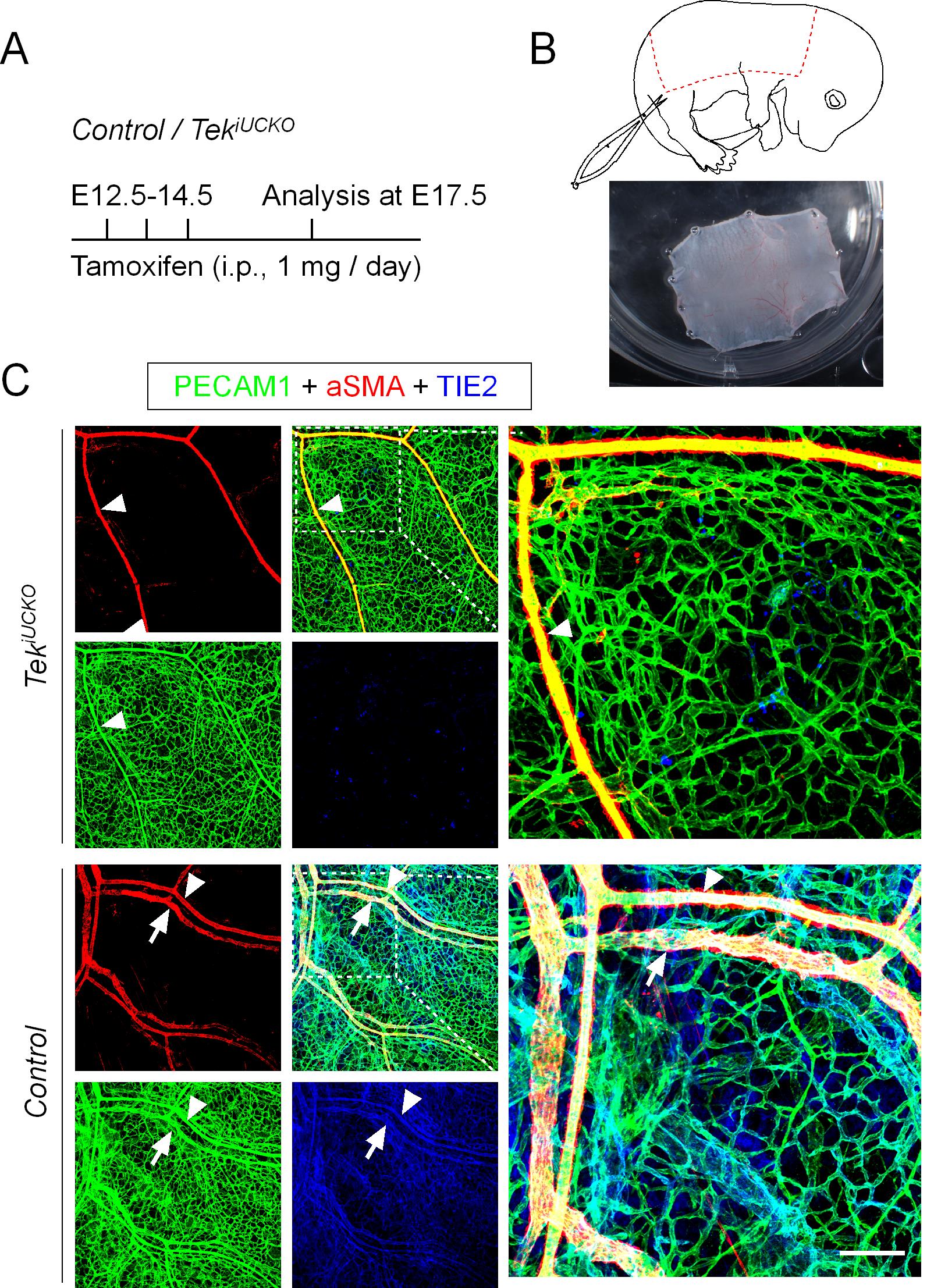
Figure 1. Disruption of cutaneous vein development following Tek deletion. (A) Tamoxifen intraperitoneal administration and analysis scheme. (B) Diagram showing the area of dorsal skin of the embryos dissected for analysis (dotted line). (C) Analysis of dorsal skin blood vessels by whole-mount immunostaining of PECAM-1 (green), αSMA (red), and TIE2 (blue) in Tek-/iUCKO (TekFlox/-; UBC-CreERT2, TekiUCKO) and control (TekFlox/+; UBC-CreERT2) mice. Note that Tek deletion was confirmed by immunostaining analysis in TekiUCKO mice. Arrowheads indicate arteries, and arrows indicate veins. Scale bar: 100 μm in C.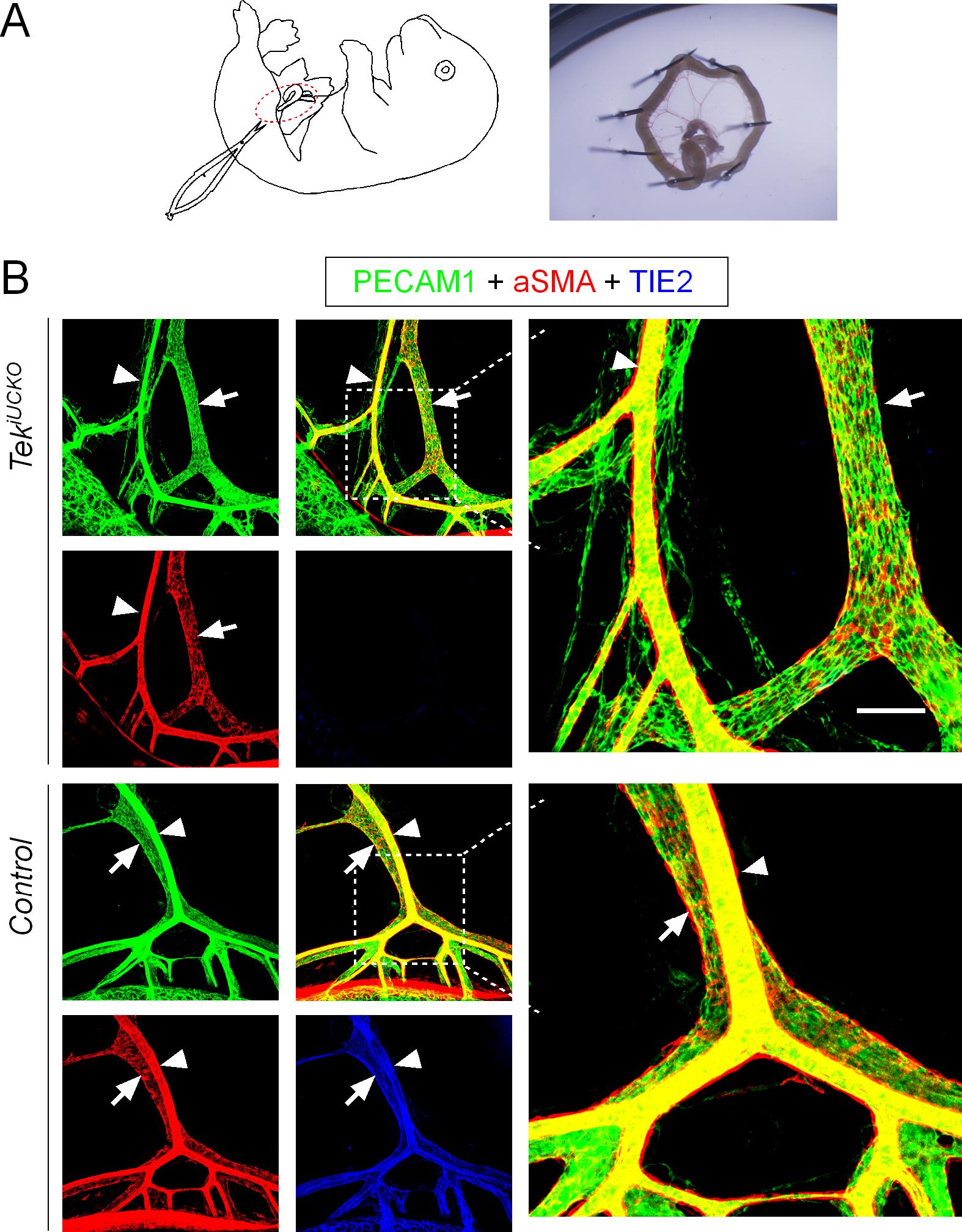
Figure 2. Misalignment of arteries and veins in the mesentery of Tek knockout mice. (A) The tamoxifen administration and analysis scheme is described in Figure 1A. Diagram showing the dissection of intestines and preparation for further analysis by fixing in a 6-well plate containing silicone rubber using fine needles. (B) Analysis of mesenteric blood vessels by whole-mount immunostaining of PECAM-1 (green), αSMA (red), and TIE2 (blue) in TekiUCKO and control mice. Arrowheads indicate arteries, and arrows indicate veins. Scale bar: 100 μm in B.
Generation of a Tek-targeted mouse model for retinal vein analysis at the neonatal stage
Note: Dissection of the retina was performed according to the protocol described by Pitulescu et al. (2010).
Administration of 30-50 μl tamoxifen solution (tamoxifen free base, 2 mg/ml in seed oil) by intragastric injection (Insulin syringe, BD Ultra-Fine®) to neonatal mice daily from postnatal day 1 to 4 (P1-4) (Figure 3A).
Euthanize the mice by cervical dislocation at P21.
Dissect the eyeballs from the mice into a 2-ml tube, and fix eyeballs in 4% PFA on ice for 2 h.
Prepare the retina by removing the cornea, sclera, choroid, pigment layer, and lens from the eye in cold PBS under a dissecting microscope.
Make four radial incisions to divide the retina into four quadrants after detaching the hyaloid vessels (Figure 3B).
Transfer the retina into a 1.5-ml tube and wash 3 times with PBS for 15 min each.
Remove PBS completely and block the retina with 3% skimmed milk powder in PBS-TX solution with gentle agitation on an orbital shaker at 4°C overnight.
Remove the blocking solution and incubate the retina with primary (PECAM-1, EphB4) and secondary antibodies at the appropriate concentrations for 12-16 h at 4°C with gentle agitation on an orbital shaker.
Note: There is a wash step between the primary and secondary antibody incubation.
Wash the retina 5 times with PBS-TX for 20 min each.
Transfer the retina onto a glass slide with a few drops of 50% glycerol and cover with a coverslip.
Analyze the retinal blood vessels under a confocal laser scanning microscope (Olympus FluoView) and acquire and process the images as described above (Figure 3C).
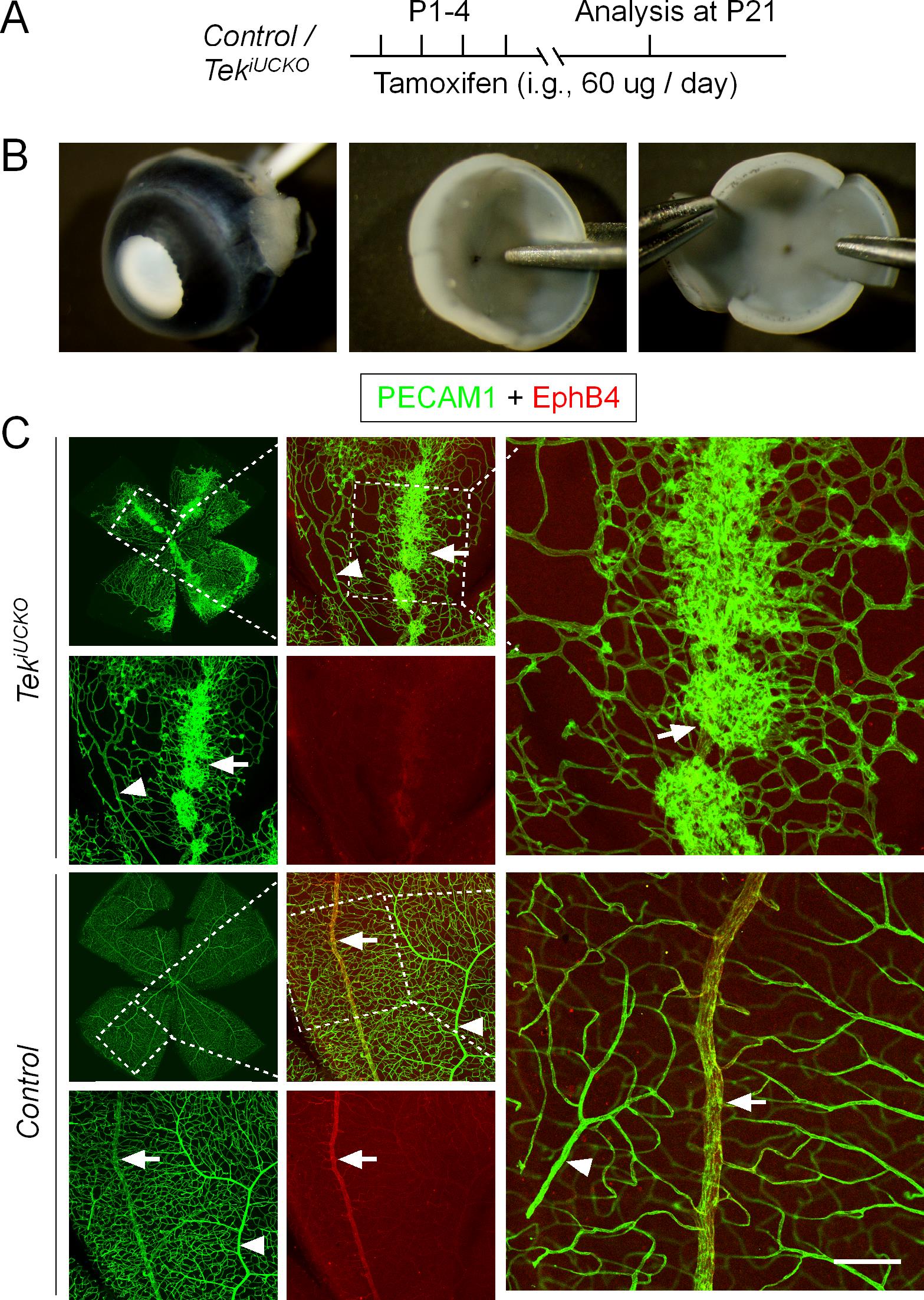
Figure 3. Formation of retinal vein-associated vascular tufts following TIE2 attenuation. (A) Tamoxifen intragastric administration and analysis scheme. (B) Mouse eyeballs were collected with the cornea, sclera, choroid, and pigment layer removed. Retinas were cut by four radial incisions for further immunohistochemical analysis. (C) Analysis of retinal blood vessels for PECAM-1 (green) and EphB4 (red) in TekiUCKO and control mice at P21. Note that TIE2 insufficiency leads to vascular tuft formation along retinal veins. Arrowheads indicate arteries, and arrows indicate veins. Scale bar: 100 μm in C.
Data analysis
In this protocol, samples were analyzed under a confocal laser scanning microscope (Olympus FluoView), and images were acquired and processed using the FV-ASW Viewer 3.0 or 4.2a software. Quantification of the retinal vascularization area and blood vascular density, which is not included in this protocol, was performed using the Image-Pro Plus 6.0 software as described in the original paper (Chu et al., 2016). We have noticed that the vascular phenotypes of Tek mutants vary depending on the Cre deleter used. For example, when VEcad-CreERT2 was used to induce Tek deletion in the postnatal studies (Okabe et al., 2014), we found that most of the Tek knockout mice (TekFlox/-; VEcad-CreERT2, TekiECKO) died before P21 (postnatal day 21, data not shown). Although Tie2 mRNA could still be detected in TekiECKO mice, this may be due to the expression of TIE2 in other cells, including hematopoietic cells, whereas TIE2 in ECs was almost undetectable (data not shown). However, almost all the TekiUCKO mice generated in this study survived even though their body weight was slightly lower than that of their littermates (Chu et al., 2016). The remaining Tie2 transcripts were analyzed by quantitative PCR. In this study, the level of Tie2 mRNA was 0.14 ± 0.05 (P7, n = 7) in the lungs of TekiUCKO mice as compared with 1.0 ± 0.16 in those of control mice (P7, n = 3).
In addition, it is noteworthy that the timepoints for tamoxifen administration and tissue analysis should be defined depending on vascular phenotypes following induced gene deletion and the specific questions to be addressed. For example, the administration of tamoxifen to pregnant mice was performed by intraperitoneal injection from E12.5 to E14.5 in this protocol as the earlier induction of Tek deletion (e.g., from E10.5) will lead to embryonic lethality at E17.5. Detailed information regarding the selection of timepoints is also described in Chu et al. (2016) (Figures 1 and 3).
Notes
Tamoxifen is light sensitive. Keep tamoxifen protected from light.
All the incubation steps should be carried out with gentle agitation on an orbital shaker.
Avoid muscle attachments when separating skin from embryos.
Keep the mesentery samples moist during confocal imaging.
Isolate eyeballs immediately after the mice have been euthanized. Avoid touching the retina with the tip of pipettes or other instruments during dissection to prevent tissue damage.
Recipes
Phosphate-buffered saline (PBS), pH 7.4
10 mM Na2HPO4
1.8 mM KH2PO4
137 mM NaCl
2.7 mM KCl
PBS-TX
0.3% Triton X-100; PBS, pH 7.4
50% glycerol
50% glycerol; PBS, pH 7.4
Acknowledgments
We thank the staff in the Animal facility of Soochow University for technical assistance. This work was supported by grants from the Ministry of Science and Technology of China (YFA0801100), the Project of State Key Laboratory of Radiation Medicine and Protection (No. GZN120 20 02), the National Natural Science Foundation of China (31970768, 81770489, 91739304), the Key Program of Natural Science Foundation of Jiangsu Higher Education Institutions (18KJA180012), the Graduate Innovation Program (KYCX19_1979), and the Priority Academic Program Development of Jiangsu Higher Education Institutions. This protocol is based on the original paper published in Elife (Chu et al., 2016).
Competing interests
The authors have no financial or non-financial competing interests to declare.
Ethics
All animal experiments were performed in accordance with the institutional guidelines of the Soochow University Animal Center (#IACUC-201611).
References
- Augustin, H. G., Koh, G. Y., Thurston, G. and Alitalo, K. (2009). Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10(3): 165-177.
- Chu, M., Li, T., Shen, B., Cao, X., Zhong, H., Zhang, L., Zhou, F., Ma, W., Jiang, H., Xie, P., Liu, Z., Dong, N., Xu, Y., Zhao, Y., Xu, G., Lu, P., Luo, J., Wu, Q., Alitalo, K., Koh, G. Y., Adams, R. H. and He, Y. (2016). Angiopoietin receptor Tie2 is required for vein specification and maintenance via regulating COUP-TFII. Elife 5: e21032.
- Deng, Y., Larrivee, B., Zhuang, Z. W., Atri, D., Moraes, F., Prahst, C., Eichmann, A. and Simons, M. (2013). Endothelial RAF1/ERK activation regulates arterial morphogenesis. Blood 121(19): 3988-3996, S3981-3989.
- Duarte, A., Hirashima, M., Benedito, R., Trindade, A., Diniz, P., Bekman, E., Costa, L., Henrique, D. and Rossant, J. (2004). Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev 18(20): 2474-2478.
- Dumont, D. J., Gradwohl, G., Fong, G. H., Puri, M. C., Gertsenstein, M., Auerbach, A. and Breitman, M. L. (1994). Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 8(16): 1897-1909.
- Lanahan, A., Zhang, X., Fantin, A., Zhuang, Z., Rivera-Molina, F., Speichinger, K., Prahst, C., Zhang, J., Wang, Y., Davis, G., Toomre, D., Ruhrberg, C. and Simons, M. (2013). The neuropilin 1 cytoplasmic domain is required for VEGF-A-dependent arteriogenesis. Dev Cell 25(2): 156-168.
- Lawson, N. D., Scheer, N., Pham, V. N., Kim, C. H., Chitnis, A. B., Campos-Ortega, J. A. and Weinstein, B. M. (2001). Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128(19): 3675-83.
- Okabe, K., Kobayashi, S., Yamada, T., Kurihara, T., Tai-Nagara, I., Miyamoto, T., Mukouyama, Y. S., Sato, T. N., Suda, T., Ema, M. and Kubota, Y. (2014). Neurons limit angiogenesis by titrating VEGF in retina. Cell 159(3): 584-596.
- Pitulescu, M. E., Schmidt, I., Benedito, R. and Adams, R. H. (2010). Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. Nat Protoc 5(9): 1518-1534.
- Ren, B., Deng, Y., Mukhopadhyay, A., Lanahan, A. A., Zhuang, Z. W., Moodie, K. L., Mulligan-Kehoe, M. J., Byzova, T. V., Peterson, R. T. and Simons, M. (2010). ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. J Clin Invest 120(4): 1217-1228.
- Sato, T. N., Tozawa, Y., Deutsch, U., Wolburg-Buchholz, K., Fujiwara, Y., Gendron-Maguire, M., Gridley, T., Wolburg, H., Risau, W. and Qin, Y. (1995). Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376(6535): 70-74.
- Vikkula, M., Boon, L. M., Carraway, K. L., 3rd, Calvert, J. T., Diamonti, A. J., Goumnerov, B., Pasyk, K. A., Marchuk, D. A., Warman, M. L., Cantley, L. C., Mulliken, J. B. and Olsen, B. R. (1996). Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell 87(7): 1181-1190.
- Wythe, J. D., Dang, L. T., Devine, W. P., Boudreau, E., Artap, S. T., He, D., Schachterle, W., Stainier, D. Y., Oettgen, P., Black, B. L., Bruneau, B. G. and Fish, J. E. (2013). ETS factors regulate Vegf-dependent arterial specification. Dev Cell 26(1): 45-58.
- You, L. R., Lin, F. J., Lee, C. T., DeMayo, F. J., Tsai, M. J. and Tsai, S. Y. (2005). Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435(7038): 98-104.
文章信息
版权信息
Cao et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Cao, X., Xu, B., Li, X., Li, T. and He, Y. (2021). A Genetically Engineered Mouse Model of Venous Anomaly and Retinal Angioma-like Vascular Malformation. Bio-protocol 11(15): e4117. DOI: 10.21769/BioProtoc.4117.
- Chu, M., Li, T., Shen, B., Cao, X., Zhong, H., Zhang, L., Zhou, F., Ma, W., Jiang, H., Xie, P., Liu, Z., Dong, N., Xu, Y., Zhao, Y., Xu, G., Lu, P., Luo, J., Wu, Q., Alitalo, K., Koh, G. Y., Adams, R. H. and He, Y. (2016). Angiopoietin receptor Tie2 is required for vein specification and maintenance via regulating COUP-TFII. Elife 5: e21032.
分类
发育生物学 > 形态建成
癌症生物学 > 通用技术 > 动物模型
医学 > 心血管疾病
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link