- EN - English
- CN - 中文
Ion Transport Activity Assay for Microbial Rhodopsin Expressed in Escherichia coli Cells
大肠杆菌表达的微生物紫红质离子转运活性测定
发布: 2021年08月05日第11卷第15期 DOI: 10.21769/BioProtoc.4115 浏览次数: 3304
评审: Laxmi Narayan MishraAnonymous reviewer(s)
Abstract
Microbial rhodopsins have diverse functions, including roles as light-driven ion pumps, light-gated ion channels, photosensors, and light-regulated enzymes. As the number of rhodopsin-like genes identified has increased in recent years, so has the requirement for rapid identification of their functions. The patch-clamp method is often used to investigate the ion transport mechanism of microbial rhodopsins in mammalian cells; however, this requires a dedicated system and advanced techniques. The ion transport assay using the Escherichia coli expression system described here evaluates the ion transport capacity by monitoring the pH change in E. coli suspensions; if the target rhodopsin has a light-dependent ion transport activity, a light-dependent pH change is observed. The pH increase or decrease corresponds to proton release from the cell or proton uptake into the cell, respectively. This method can be used to evaluate ion transport capacity in a high-throughput manner using a combination of general-purpose equipment and common techniques.
Graphic abstract:
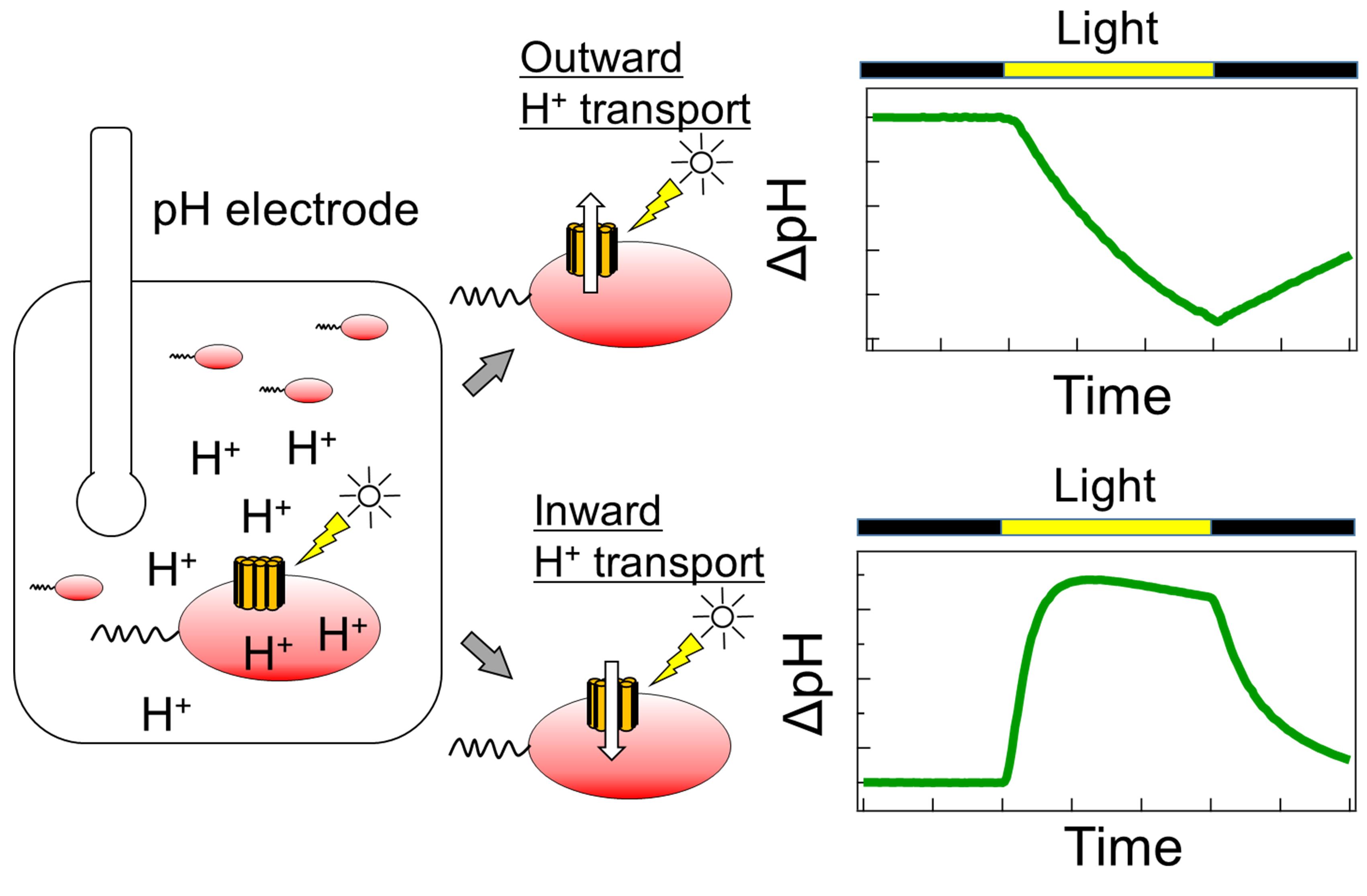
Schematic diagram of the ion transport assay in rhodopsin-expressing E. coli cells.
Background
Microbial rhodopsins are light-perceptive membrane proteins containing all-trans retinylidine chromophores, known as retinal. These proteins are found in many organisms, including bacteria, fungi, archaea, and giant viruses (Ernst et al., 2014). With the accumulation of genetic information, the number of genes predicted to be microbial rhodopsins is rapidly increasing; however, many of their molecular properties remain unknown.
Microbial rhodopsins have various functions, including roles as light-driven ion pumps, light-gated ion channels, photosensors, and light-regulated enzymes. In particular, rhodopsins that show ion transport activity are attracting attention for their use as optogenetic tools for the optical control of neural activity; therefore, rapid progress in gene screening is required. The patch-clamp method is often used to investigate the ion transport function and mechanism of microbial rhodopsin in mammalian cells (Klapoetke et al., 2014). In addition, the proteoliposome, into which purified rhodopsin protein is reconstituted, is used to measure ion transport (Waschuk et al., 2005); however, these methods require dedicated equipment and advanced techniques.
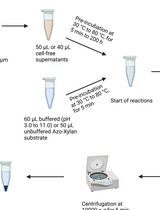
Simpler ion transport assays using rhodopsin-expressing Escherichia coli cells can also be used to evaluate ion transport capacity. In this method, rhodopsin-expressing cells are suspended in a salt solution, such as NaCl, KCl, or Na2SO4, and the light-dependent pH change in the cell suspension is subsequently monitored. This light-dependent pH change is caused by H+ transport across the plasma membrane, which is coupled with ion transport by rhodopsin. In contrast to the patch-clamp method, this method can be used to evaluate ion transport capacity in a high-throughput manner using general-purpose equipment, without the requirement for specialized instrumentation or expertise. In fact, this method has contributed to the discovery of novel microbial rhodopsins with various ion transport capacities (Inoue et al., 2013, 2016, 2020; Yoshizawa et al., 2014; Harris et al., 2015; Hasemi et al., 2016; Pushkarev and Béjà, 2016; Needham et al., 2019). The details of this method are described herein.
Materials and Reagents
Disposable pipette tips, 0.1-10 μl (M&S Instrument, catalog number: F161630)
Disposable pipette tips, 2.0-200 μl (M&S Instrument, catalog number: F161930)
Disposable pipette tips, 100-1,000 μl (M&S Instrument, catalog number: F161670)
Disposable pipette tips, 500-5,000 μl (M&S Instrument, catalog number: F161571)
Glass test tubes, 18 × 150 mm
Aluminum caps for glass tubes
300-ml shaking Erlenmeyer flasks with baffles
Silicone stoppers (AS ONE, BIO-SILICO®, N-42, catalog number: 5-1100-03)
Polypropylene conical centrifuge tubes (Thermo Fisher Scientific, catalog number: 3009650)
Disposable cells for near-ultraviolet and visible light (AS ONE, Standard Type, catalog number: 1-2848-01)
Sterile syringe filters, 0.2-μm (Pall, Acrodisc®, Product ID: 4612)
E. coli C43 (DE3) cells (Lucigen, OverExpressTM strain, catalog number: 60446-1)
Target microbial rhodopsin gene cloned into the pET21a(+) vector
Note: The target gene was optimized for its codon for E. coli and synthesized (GenScript). The synthesized gene was cloned between the NdeI and XhoI sites in the pET21a(+) vector (Sigma-Aldrich, Novagen, catalog number: 69740).
Yeast extract (BD, catalog number: 212750)
Tryptone (Nacalai Tesque, catalog number: 35640-95)
Sodium chloride (FUJIFILM Wako Pure Chemical, catalog number: 191-01665)
Ampicillin sodium (FUJIFILM Wako Pure Chemical, catalog number: 014-23302)
Antifoam PE-L (FUJIFILM Wako Pure Chemical, catalog number: 013-17201)
Isopropyl-β-D-thiogalactoside (IPTG; Sigma-Aldrich, catalog number: I5502)
All-trans-retinal (Toronto Research Chemicals, catalog number: R240000)
Hydrochloric acid (FUJIFILM Wako Pure Chemical, catalog number: 080-01066)
Sodium hydroxide (FUJIFILM Wako Pure Chemical, catalog number: 192-15985)
Carbonyl-cyanide 3-chlorophenylhydrazone (CCCP) (Sigma-Aldrich, catalog number: C2759)
Dimethyl sulfoxide (DMSO) (FUJIFILM Wako Pure Chemical, catalog number: 046-21981)
Disodium hydrogenphosphate (FUJIFILM Wako Pure Chemical, catalog number: 042-30055)
Hydroxylamine hydrochloride (FUJIFILM Wako Pure Chemical, catalog number: 081-01471)
n-Dodecyl-β-D-maltoside (DDM) (Anatrace, catalog number: D310)
Lysozyme from egg white (FUJIFILM Wako Pure Chemical, catalog number: 129-06723)
Deoxyribonuclease I, from bovine pancreas, precrystalline (DNaseI) (FUJIFILM Wako Pure Chemical, catalog number: 043-26773)
2× YT medium (see Recipes)
50 mg/ml ampicillin stock solution (see Recipes)
30 mM CCCP stock solution (see Recipes)
2 M hydroxylamine stocks (see Recipes)
Bleaching buffer (see Recipes)
Equipment
Constant-temperature shaking incubator (TAITEC, Bioshaker BR-43FL·MR, Code number: 0053027-000)
High-speed refrigerated centrifuge (Eppendorf Himac Technologies, model: CF15RN)
Fixed-angle rotor (Eppendorf Himac Technologies, model: T15A42)
Micropipettes (10- and 200-μl, and 5-ml; Gilson)
Vortex mixer (Electro Scientific Industries, model: GENIE2, catalog number: SI-0286)
Digital colorimeter (TAITEC, model: miniphoto518R, catalog number: 0040889-000)
Tube rotator (AS ONE, model: ACR-100)
pH meter (HORIBA, LAQUA, model: F-55)
pH electrode (HORIBA, model: 9618S-10D)
Benchtop darkroom
pH electrode cover for light protection
Note: We made a cover for the pH electrode by cutting the bottom of a 15-ml tube and covering it with black cloth tape.
Water-jacketed glass cell vial
Magnetic stirrer (AS ONE, model: CT-MINI)
Low-temperature circulating bath (EYELA, model: NCB-1210)
300-W xenon arc light source (ASAHI spectra, model: MAX-303)
Long-pass filter (AGC Techno Glass, model: Y-52)
Heat-absorbing filter (SIGMAKOKI, model: HAF-50S-50H)
Ultrasonic homogenizer (TAITEC, model: VP-300N)
UV-visible spectrometer (JASCO, model: V-750)
Integral sphere unit for a UV-visible spectrometer (JASCO, model: ISV-922)
Suction pump (AIR LIQUIDE, model: SP30)
Software
Data collection software for a pH meter (HORIBA)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Konno, M., Inoue, K. and Kandori, H. (2021). Ion Transport Activity Assay for Microbial Rhodopsin Expressed in Escherichia coli Cells. Bio-protocol 11(15): e4115. DOI: 10.21769/BioProtoc.4115.
- Inoue, K., Tsunoda, S. P., Singh, M., Tomida, S., Hososhima, S., Konno, M., Nakamura, R., Watanabe, H., Bulzu, P. A., Banciu, H. L., Andrei, A. S., Uchihashi, T., Ghai, R., Beja, O. and Kandori, H. (2020). Schizorhodopsins: A family of rhodopsins from Asgard archaea that function as light-driven inward H+ pumps. Sci Adv 6(15): eaaz2441.
分类
微生物学 > 微生物生理学 > 离子迁移
生物物理学 > 电生理
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link