- EN - English
- CN - 中文
A Swimming-based Assay to Determine the Exercise Capacity of Adult Zebrafish Cardiomyopathy Models
基于游泳的测定成年斑马鱼心肌病模型运动能力的方法
发布: 2021年08月05日第11卷第15期 DOI: 10.21769/BioProtoc.4114 浏览次数: 2942
评审: William C. W. ChenJohn W PetersonAnonymous reviewer(s)
Abstract
Exercise capacity, measured by treadmill in humans and other mammals, is an important diagnostic and prognostic index for patients with cardiomyopathy and heart failure. The adult zebrafish is increasingly used as a vertebrate model to study human cardiomyopathy due to its conserved cardiovascular physiology, convenience for genetic manipulation, and amenability to high-throughput genetic and compound screening. Owing to the small size of its body and heart, new phenotyping assays are needed to unveil phenotypic traits of cardiomyopathy in adult zebrafish. Here, we describe a swimming-based functional assay that measures exercise capacity in an adult zebrafish doxorubicin-induced cardiomyopathy model. This protocol can be applied to any adult zebrafish model of acquired or inherited cardiomyopathy and potentially to other cardiovascular diseases.
Graphic abstract:
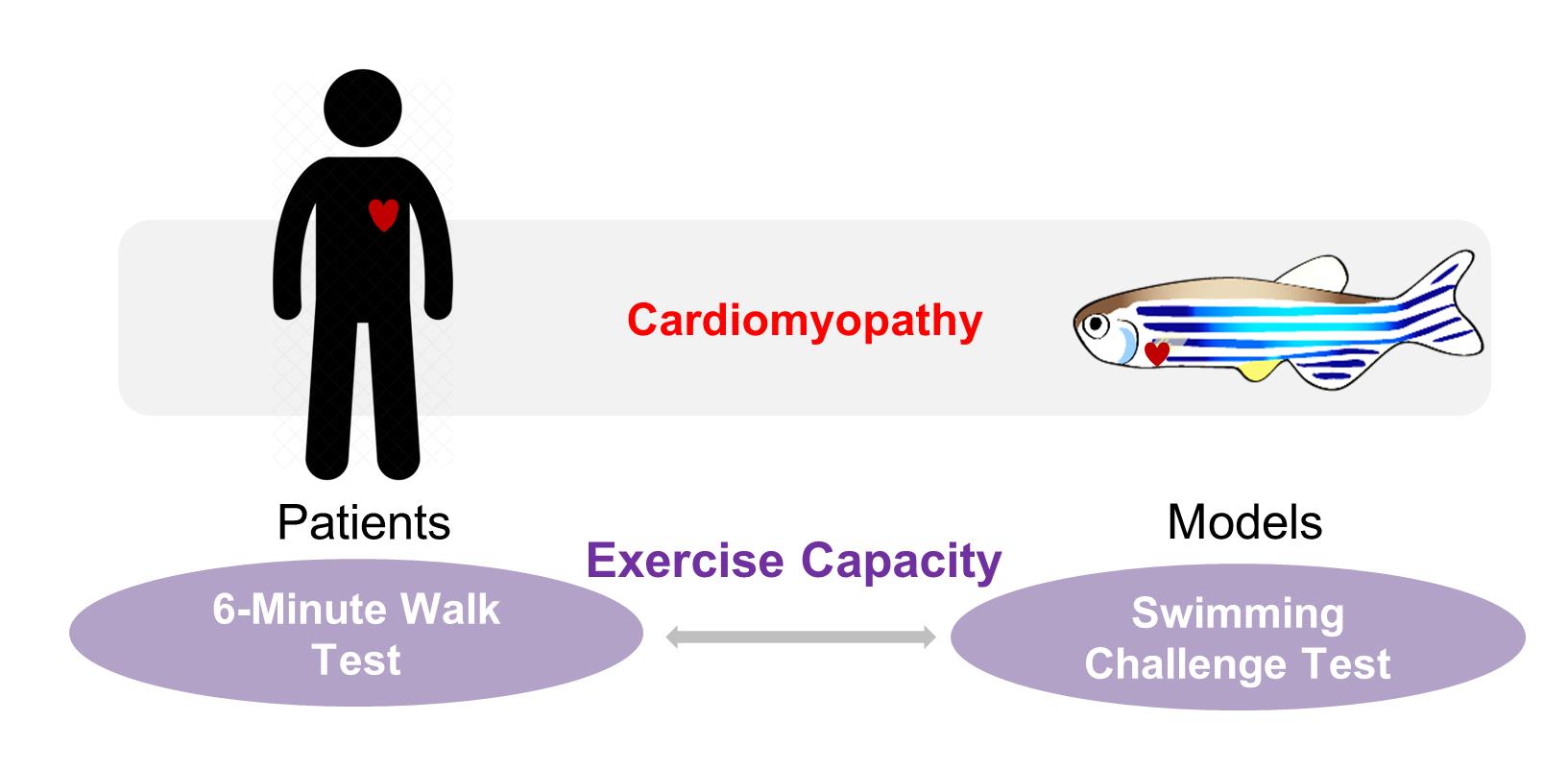
Clinical relevance of the swimming-based phenotyping assay in adult zebrafish cardiomyopathy models.
Background
Given its conserved cardiovascular physiology and genetic amenability, adult zebrafish is an emerging vertebrate model for cardiomyopathy studies (Gut et al., 2017). Since the first evidence of conserved cardiac remodeling processes in this low vertebrate model (Sun et al., 2009), a panel of acquired and inherited cardiomyopathy models has been established in adult zebrafish (Dvornikov et al., 2018). In comparison with rodent models, zebrafish models possess higher throughput that enables forward genetic screens to identify novel genetic modifiers (Ding et al., 2016; Ma et al., 2018) and chemical screens to rapidly assess therapeutic drug candidates for cardiomyopathy. However, phenotyping tools for adult zebrafish models of cardiomyopathy remain a bottleneck, partially due to technical challenges associated with the small size of adult zebrafish.
In human patients with cardiomyopathy and subsequent heart failure, exercise intolerance is an important pathophysiologic hallmark (Del Buono et al., 2019). For example, the stratification of heart failure patients following the New York Heart Association (NYHA) classification guidelines is based heavily on exercise capacity. The 6-min walk test, in particular, is a classic and commonly used quantitative method to determine exercise capacity in clinical settings, which has been used to provide important diagnostic and prognostic information in cardiomyopathy and heart failure patients (Pina et al., 2003; Del Buono et al., 2019). To develop a corresponding phenotyping tool for adult zebrafish models, we recently leveraged a swimming-based assay to evaluate the exercise capacity in a doxorubicin-induced cardiomyopathy (DIC) model (Ma et al., 2020). We found that the critical swimming speed is a useful non-invasive index to validate the successful establishment of the model and evaluate the benefits of therapeutic intervention. Indeed, the swimming assay has also been implemented in studies using zebrafish as models for a variety of purposes including tissue regeneration (Wang et al., 2011; Mokalled et al., 2016), skeletal muscle growth (Palstra et al., 2010), and aging (Gilbert et al., 2014). More recently, a general protocol to measure the critical swimming speed in adult zebrafish has been described in detail (Wakamatsu et al., 2020). Herein, we describe the protocol used in our program for phenotyping an adult zebrafish model of DIC (Ding et al., 2011; Ma et al., 2018). It is anticipated that this protocol can be extended to other adult zebrafish models of cardiovascular diseases (Ding et al., 2020).
Materials and Reagents
Zebrafish WIK strain (In house)
Zebrafish housing system water (In house)
Hank’s Balanced Salt Solution (ThermoFisher Scientific, catalog number: 14025092)
Doxorubicin hydrochloride (Sigma-Aldrich, catalog number: D1515)
Ethyl 3-aminobenzoate methanesulfonate, Tricaine (Sigma-Aldrich, catalog number: E10521)
Equipment
Swim tunnel (Loligo Systems, model: 10L 120V/60Hz, catalog number: SW10110)
Aquarium air pump (API, Rena Air Pump, model 400)
Metal sheets (11.2 × 9.5 cm; pore diameter: 0.25-0.50 cm; made of steel; see Figure 1A)
Water flow meter (Hontzsch, flowtherm NT)
Weight scale (Torbal, catalog number: AD520)
Timer (Fisher Scientific, Fisherbrand, catalog number: 14-649-17)
Software
Excel (Microsoft Office) or JMP (SAS Institude Inc.)
Prism (GraphPad)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ma, X. and Xu, X. (2021). A Swimming-based Assay to Determine the Exercise Capacity of Adult Zebrafish Cardiomyopathy Models . Bio-protocol 11(15): e4114. DOI: 10.21769/BioProtoc.4114.
- Ma, X., Zhu, P., Ding, Y., Zhang, H., Qiu, Q., Dvornikov, A. V., Wang, Z., Kim, M., Wang, Y., Lowerison, M., Yu, Y., Norton, N., Herrmann, J., Ekker, S. C., Hsiai, T. K., Lin, X. and Xu, X. (2020). Retinoid X receptor alpha is a spatiotemporally predominant therapeutic target for anthracycline-induced cardiotoxicity. Sci Adv 6(5): eaay2939.
分类
医学 > 心血管疾病
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










