- EN - English
- CN - 中文
Cell-attached and Whole-cell Patch-clamp Recordings of Dopamine Neurons in the Substantia Nigra Pars Compacta of Mouse Brain Slices
小鼠脑片黑质致密部多巴胺神经元的细胞贴附和全细胞膜片钳记录
发布: 2021年08月05日第11卷第15期 DOI: 10.21769/BioProtoc.4109 浏览次数: 5702
评审: Giusy TornilloMarco Pagliusi Jr.Xiaoliang Zhao
Abstract
The Substantia Nigra pars compacta (SNc) is a midbrain dopaminergic nucleus that plays a key role in modulating motor and cognitive functions. It is crucially involved in several disorders, particularly Parkinson’s disease, which is characterized by a progressive loss of SNc dopaminergic cells. Electrophysiological studies on SNc neurons are of paramount importance to understand the role of dopaminergic transmission in health and disease. Here, we provide an extensive protocol to prepare SNc-containing mouse brain slices and record the electrical activity of dopaminergic cells. We describe all the necessary steps, including mouse transcardiac perfusion, brain extraction, slice cutting, and patch-clamp recordings.
Keywords: Acute brain slices (急性脑片)Background
The Substantia Nigra pars compacta (SNc) is a midbrain nucleus that provides a strong dopaminergic input to various areas of the brain, including the basal ganglia (BG) (Gerfen and Bolam, 2010). Dopaminergic axons originate from tyrosine hydroxylase (TH)-expressing neurons of the SNc. The neuromodulator dopamine (DA) released by synaptic terminals of these projections provides a vital contribution to the regulation of BG microcircuitry, which is crucially involved in modulating motor and reward functions (Zhai et al., 2019).
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease. Dopaminergic neurons degenerate in PD, leading to a depletion of dopamine in the striatal circuitry and causing a detrimental imbalance between direct and indirect output pathways (Gerfen and Surmeier, 2011). This ultimately results in devastating symptoms such as persistent tremors, bradykinesia, rigidity, and often neuropsychiatric alterations (Surmeier et al., 2017). Thus, advancing our knowledge of dopaminergic neuronal functions in normal and pathological conditions is a task of great importance and a major goal for many neuroscience laboratories.
Although primary cultures are an interesting system to investigate the cellular physiology of dopaminergic neurons, a relatively low yield of about 5% in tyrosine hydroxylase (TH, a cytochemical marker of DA cells)-expressing neurons may represent a challenge for electrophysiological experiments (Choi et al., 2013). Moreover, it has been reported that cultured dopaminergic neurons lose their autonomous pacemaking activity (Masuko et al., 1992), which is crucial for the sustained release of dopamine and plays a fundamental role in both physiological and pathological conditions. On the other hand, ex vivo brain slices represent a reliable and reproducible preparation preserving specific features of dopaminergic neurons, including their autonomous pacemaking activity. Yet, a potential downside of this technique is the deafferentation caused by the cutting process used to prepare the sample. Alternative techniques such as in vivo extracellular single-unit recordings could be used to study TH+ neurons (Farassat et al., 2019); however, whole-cell recordings may be difficult to obtain in vivo, precluding the possibility to investigate intrinsic and synaptic properties of DA cells. Slice experiments therefore remain the gold-standard option for whole-cell recordings in DA neurons.
A great number of studies provide combinations of slice cutting and recording solutions that are specifically used for different brain regions (Bischofberger et al., 2006) from young, adult, or aging mice (Ting et al., 2014). Technical and manual precautions are obviously necessary in order to avoid, or at least minimize, various types of insults (mechanical, osmotic, metabolic, etc.) inflicted to the brain specimen during slice preparation. In particular, sucrose-based artificial cerebrospinal fluid (ACSF) has been used to prevent excessive intracellular influx of chloride and subsequent cell swelling and lysis (Aghajanian and Rasmussen, 1989). Here, we provide a sucrose-based cutting solution and slice preparation protocol tailored to SNc-containing brain slices. We describe all the necessary steps, including transcardiac perfusion, brain extraction, dissection, and patch-clamp recordings. We show how these methods can be used to study the pharmacological effects of acutely applied drugs as a tool to investigate potential novel targets for the treatment of neurodegenerative disorders such as PD (Regoni et al., 2020).
Materials and Reagents
NaCl (Sigma-Aldrich, catalog number: 793566; store at room temperature (RT))
KCl (Sigma-Aldrich, catalog number: 409316; RT)
NaH2PO4 (Sigma-Aldrich, catalog number: S5011; RT)
CaCl2 1 M (Fluka, catalog number: 21114; store at 4°C)
NaHCO3 (Sigma-Aldrich, catalog number: 792519; RT)
MgCl2 (Sigma-Aldrich, catalog number: M8266; RT)
D-glucose (Sigma-Aldrich, catalog number: G5767; RT)
Sucrose (Sigma-Aldrich, catalog number: S9378; RT)
KH2PO4 (Sigma-Aldrich, catalog number: P9791; RT)
HEPES (Sigma-Aldrich, catalog number: H4034; RT)
EGTA (Sigma-Aldrich, catalog number: 03777; RT)
Na2-ATP (Sigma-Aldrich, catalog number: A7699; store at -20°C)
Na-GTP(Sigma-Aldrich, catalog number: G8877; store at -20°C)
KOH (Fluka, catalog number: 319376; store at 4°C)
NaOH (Sigma-Aldrich, catalog number: S2770; store at 4°C)
Agarose (Fisher Scientific, catalog number: BP1356; RT)
Ketamine-HCl (Anesketin (100 mg/ml), Dechra; RT)
Xylazine (Rompun (20 mg/ml), Bayer; RT)
Ketamine/xylazine anaesthetic mixture (see Recipes)
Artificial cerebrospinal fluid (ACSF) 10× stock solution (w/o CaCl2 and MgCl2; see Recipes)
MgCl2 1 M stock solution (see Recipes)
Artificial cerebrospinal fluid (ACSF) 1× (see Recipes)
Cutting solution (see Recipes)
Internal solution (see Recipes)
Equipment
Equipment for brain slice preparation
VT1000S vibratome (Leica Microsystems, Wetzlar, Germany)
Osmometer 3320 (Advanced Instruments, Norwood, MA)
TW2 thermostatic water bath (Julabo, Seelbach, Germany)
Peristaltic pump with perfusion tubing line (Gilson Minipuls 3)
Paper filter disk (Millipore, catalog number: GSWPP04700)
Glass Petri dish (diameter: 10 cm; Merck, catalog number: BR455701)
Gas diffusing stone (Fisher Scientific, catalog number: 10686185)
50-ml beaker
Stainless steel vibratome blades (Campden Instruments, catalog number: 752/1/SS)
Equipment for custom-made slice chamber
Plastic Petri dish (Thermo Fisher Scientific, catalog number: 150318)
Nylon mesh
Cyanoacrylate glue (e.g., Super Attack; Loctite)
100-ml beaker
Borosilicate glass capillaries (Sutter Instrument, catalog number: BF100-50-7.5 or equivalents)
Two plastic Pasteur pipettes
Assembly: The maintenance chamber is made up of a plastic Petri dish (diameter 3.5 cm), in which the bottom surface has been substituted with a soft nylon mesh (Figure 1A). The mesh is fixed to the dish rim with cyanoacrylate glue. The chamber is positioned inside a 100-ml beaker containing ACSF (70 ml) (Figure 1C). A glass capillary attached to a gas-impermeable tube is placed inside the chamber to supply oxygen (95% O2 + 5% CO2) (Figure 1D). The heads of two plastic Pasteur pipettes, one embedded into the other, are used to shim the chamber and hold the capillary inside the beaker (Figure 1B,C), to avoid bubble diffusion directly onto the slices. During preparation, individual slices are gently positioned on the mesh immediately after being cut away from the brain. After use, the maintenance chamber should be regularly disassembled for careful cleaning or replacement of its components.
Dissection tools
Surgical scissors, sharp blunt (e.g., Fine Science Tools (FST), catalog number: 14001-14)
Fine scissors, sharp 11.5 cm (e.g., FST, catalog number: 14060-11)
Fine scissors, sharp 10.5 cm (e.g., FST, catalog number: 14060-10)
Dumont #5, fine forceps (e.g., FST, catalog number: 11254-20)
Dumont #3c, forceps (e.g., FST, catalog number: 11231-20)
Blunt forceps (Aven Forceps, Straight Serrated Tips, catalog number: 18433)
Vannas spring scissors (e.g., FST, catalog number: 15018-10)
Disposable sterile scalpel blade No.21
Spoon (similar to VWR, catalog number: USBE3317)
Flat spatula (similar to VWR, catalog number: RSGA038.185)
Cyanoacrylate glue (“superglue,” e.g., Super Attack, Loctite)
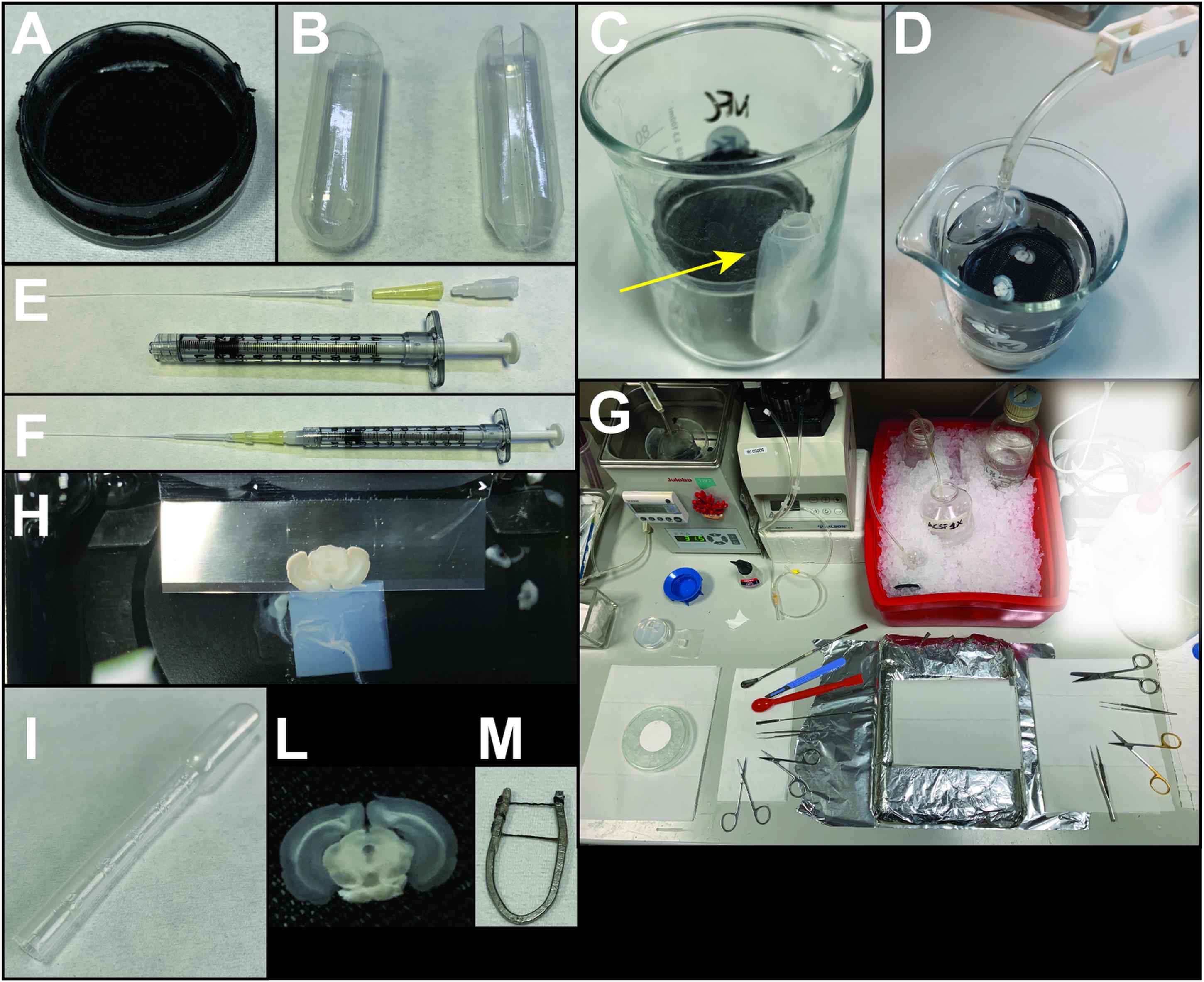
Figure 1. Representative images of the equipment required for SNc brain slice preparation. (A) Maintenance chamber. (B) Heads of plastic Pasteur pipettes used to create the envelope for the glass capillary oxygenator (arrow in C). (C, D) Custom-made slice chamber. (E-F) Components and assembled syringe for back-filling the electrode (see Note 4). (G) Preparation of the perfusion/slicing desk. (H) Coronal slice cut by the vibratome blade. Note the agarose cube positioned behind the brain specimen. (I) Plastic Pasteur pipette used to handle the brain slices. (L) Example of an SNc-containing brain slice. (M) U-shaped custom-made anchor (see Note 3).
Equipment for electrophysiology experiments (Figure 2)
BX51WI upright microscope (Olympus, Japan) equipped with infrared-differential interference contrast optics (IR-DIC)
IsoStation anti-vibration table (Newport, Irvine, CA)
Charge-coupled device (CCD) camera (e.g., Hamamatsu, QImaging, Sentech, Teledyne, etc.)
MultiClamp 700B operational amplifier (Molecular Devices, Sunnyvale, CA)
Digidata 1440A digitizer (Molecular Devices, Sunnyvale, CA)
Personal computer
Mini 25 micromanipulator unit (Luigs & Neumann)
SM-5 electronic control (Luigs & Neumann)
Badcontroller V temperature controller (Luigs & Neumann)
PC-10 electrode puller (Narishige, Japan)
1-ml BD Luer-LokTM insulin syringe without needle (BD, catalog number: 329651)
Microloader tips (Eppendorf, catalog number: 5242956003)
Syringe-driven filter unit (PVDF, 0.22 μm; SLGVR04NL Millex)
Home-made brain U-shaped anchor (see Note 3)
Borosilicate glass capillaries with filament (O.D.: 1.5 mm, I.D.:1.10 mm; 7.5 cm length) (Sutter Instrument, catalog number: BF150-110-7.5HP)

Figure 2. Patch-clamp setup
Software
pClamp 10 (Molecular Devices, https://www.moleculardevices.com)
GraphPad Prism (La Jolla, CA, https://www.graphpad.com/scientific-software/prism/)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Cattaneo, S., Regoni, M., Sassone, J. and Taverna, S. (2021). Cell-attached and Whole-cell Patch-clamp Recordings of Dopamine Neurons in the Substantia Nigra Pars Compacta of Mouse Brain Slices. Bio-protocol 11(15): e4109. DOI: 10.21769/BioProtoc.4109.
分类
细胞生物学 > 基于细胞的分析方法 > 电生理技术
神经科学 > 基础技术 > 快速制片
生物物理学 > 电生理 > 膜片钳技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link