- EN - English
- CN - 中文
Isolation and in vitro Culture of Mouse Oocytes
小鼠卵母细胞的分离与体外培养
(*contributed equally to this work) 发布: 2021年08月05日第11卷第15期 DOI: 10.21769/BioProtoc.4104 浏览次数: 7414
评审: Chiara AmbrogioAnonymous reviewer(s)
Abstract
Females are endowed at birth with a fixed reserve of oocytes, which declines both in quantity and quality with advancing age. Understanding the molecular mechanisms regulating oocyte quality is crucial for improving the chances of pregnancy success in fertility clinics. In vitro culture systems enable researchers to analyse important molecular and genetic regulators of oocyte maturation and fertilisation. Here, we describe in detail a highly reproducible technique for the isolation and culture of fully grown mouse oocytes. We include the considerations and precautionary measures required for minimising the detrimental effects of in vitro culture conditions. This technique forms the starting point for a wide range of experimental approaches such as post-transcriptional gene silencing, immunocytochemistry, Western blotting, high-resolution 4D time-lapse imaging, and in vitro fertilization, which are instrumental in dissecting the molecular determinants of oocyte quality. Hence, this protocol serves as a useful, practical guide for any oocyte researcher beginning experiments aimed at investigating important oocyte molecular factors.
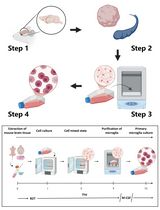
Graphic abstract:

A step-by-step protocol for the isolation and in vitro culture of oocytes from mice.
Background
Oocytes provide the overwhelming majority of cytoplasmic building blocks required by the embryo and must undergo two rounds of error-free chromosome segregation to ensure normal embryonic development (Chaigne et al., 2017; Greaney et al., 2017). The first of these divisions, meiosis I (MI), is notoriously error-prone, especially with advancing female age; indeed, errors arising during oocyte MI account for a staggering 80-90% of human aneuploidy (Greaney et al., 2017). MI is also the division in which symmetry-breaking occurs to enable the oocyte to expel half of its chromosomes into a very small daughter cell (known as the polar body, PB), whilst holding on to the bulk of the cytoplasm to support later embryonic development (Chaigne et al., 2017). It is well established that oocyte numbers, ovulation, and oocyte quality are the three oocyte-specific parameters that determine fertility and pregnancy success (Homer, 2020). While there are specific biomarkers and diagnostic tools to test oocyte numbers and ovulation, there is no definitive diagnostic test for oocyte quality. Although human oocytes would be the ideal research material for investigating oocyte quality, extensive restrictions and ethical concerns have made them almost completely inaccessible for experimentation. Hence, the molecular basis of oocyte quality remains poorly understood and will benefit from more in-depth investigation of key aspects such as meiotic maturation, fertilisation, and embryonic development. Interrogating the roles of genes that regulate these processes has been accomplished using surrogate mammalian models such as the mouse, which has become the most widely studied model.
Breakthrough advancements in understanding key molecular regulators of MI chromosome segregation and asymmetrical division, as well as the oocyte’s vulnerability to aging, have been dependent on the ability to perform detailed analyses of oocytes in vitro. The applications of this protocol are wide-ranging and allow for post-transcriptional gene silencing (for example, using RNAi and morpholinos), conventional biochemical analyses such as Western blotting, and in-depth visual interrogation of intracellular structures by immunocytochemistry and time-lapse microscopy. Our laboratory has used all these techniques during in vitro culture to uncover numerous unique facets of oocyte regulation, such as spindle assembly checkpoint (SAC) regulation of MI chromosome segregation and its decline with aging (Homer et al., 2005; Gui and Homer, 2012; Riris et al., 2014), a unique oocyte DNA damage response (Subramanian et al., 2020) and new genetic insight into DNA damage-based premature ovarian aging (Subramanian et al., 2021), novel aspects of regulation at the G2-M boundary (Homer et al., 2009; Gui and Homer, 2013), the contribution of late post-anaphase events to asymmetrical division (Wei et al., 2018 and 2020), and key metabolic nodes involved in oocyte aging and potential therapeutics targeting these nodes to reverse poor oocyte quality (Bertoldo et al., 2020; Iljas and Homer, 2020; Iljas et al., 2020).
There are a number of challenges involved in studying oocytes. One major challenge is that oocytes cannot be propagated in the lab like many immortal cell lines and must be isolated as primary cells each time they are required for experimentation. Another challenge is the very long duration of MI, which lasts 6-12 h depending on the mouse strain (versus minutes for mitosis), and requires lengthy experiments. Yet another challenge is the large volume of the oocyte (~40-times larger than that of a somatic cell), which makes the identification of small cellular structures markedly more difficult. It is therefore critically important to develop consistent and reproducible techniques for oocyte isolation and culture.
Oocyte culture methods were first developed as early as the 1930’s (Moricard and de Fonbrune, 1937). Later, experiments developed a simple isotonic solution capable of sustaining oocyte maturation (Donahue, 1968 and 1970), which allowed oocytes to be observed during MI for the first time. More sophisticated culture media were subsequently developed (Chatot et al., 1989), and now, oocyte isolation and culture protocols form the basis of all in vitro techniques used for studying oocyte biology. Here, we detail a method involving hormonal stimulation and collection of ovaries, followed by isolation and in vitro culture of oocytes.
In this protocol, pregnant mare serum gonadotrophin (PMSG) is used to stimulate antral follicle development, thereby increasing the yield of oocytes isolated from the ovary and enabling 30-100 oocytes to be obtained from each mouse depending on the strain and age (Wei et al., 2018 and 2020; Subramanian et al., 2020 and 2021). By minimising exposure to light and temperature fluctuations, this method reduces both the damage caused by in vitro culture and the resulting artefacts.
This protocol describes the collection of fully grown oocytes arrested in prophase of MI, characterised by the presence of an intact nucleus, which in oocytes is referred to as the germinal vesicle (GV). Prophase I-arrest can be experimentally maintained using chemicals such as 3-isobutyl-1- methylxanthine (IBMX), which sustains high cyclic adenosine monophosphate (cAMP) levels, thereby preventing activation of the major M phase-promoting kinase, cyclin-dependent kinase 1 (Cdk1) (Zhao et al., 2014). Conversely, resumption of meiotic maturation can easily be induced by washing out IBMX, thereby providing a reversible method for manipulating M-phase entry. This approach also allows for the timed introduction of small molecule inhibitors to disrupt regulators at any number of key timepoints during the cell cycle.
The applications of this protocol are extensive and have been critical for advancing our understanding of the regulation of MI.
Materials and Reagents
Cell culture dishes; 35 × 10 mm and 60 × 15 mm (Sigma-Aldrich, catalog numbers: CLS430165, CLS 430166)
Syringe filter unit; 0.22-µm (Merck, catalog number: SLGP033RS)
Microcentrifuge tubes; 0.6-ml and 1.6-ml (Neptune, catalog numbers: 3735.X, 3745.X)
Centrifuge tubes; 15-ml and 50-ml (Corning, catalog numbers: 352096, 352070)
Disposable ultra-fine needles (27 G × 1/2”; Hanke Sass Wolf, catalog number: 4710004012) and 1-ml syringes (Terumo, catalog number: SS+01T Tuberculin)
Glass Pasteur pipettes (230 mm) (VWR, catalog number: 6121702)
Mouth aspirator tube assembly (Sigma-Aldrich, catalog number: A5177)
Mice (>4 week-old females; Strains used: B6CBF1) (University of Queensland Biological Resources, University of Queensland, Australia)
PMSG (Prospec, catalog number: HOR-272)
Bovine serum albumin (BSA; Sigma-Aldrich, catalog number: A7096)
Light mineral oil suitable for mouse embryo culture (Sigma-Aldrich, catalog number: M5310)
IBMX (Sigma-Aldrich, catalog number: I5879)
MilliQ water
Sodium bicarbonate (Sigma-Aldrich, catalog number: S5761)
HEPES (Free acid) (Sigma-Aldrich, catalog number: H3375)
HEPES (Salt) (Sigma-Aldrich, catalog number: H7006)
Gentamicin sulfate (Sigma-Aldrich, catalog number: G1264)
M16 medium (Sigma-Aldrich, catalog number: M7292) for in vitro culture
α-Minimum Essential Medium (MEM) powder (Thermo Fisher Scientific, catalog number: 12000022) (see Recipes)
Equipment
Fine dissecting scissors (Met-App Scientific and Surgical Instruments, catalog number: 2235)
Fine forceps (Met-App Scientific and Surgical Instruments, catalog number: 2127)
Stereomicroscope (Leica Microsystems, model: M165C; Nikon Microscope Solutions, model: SMZ800N)
Dry heating block (Grant Instruments, model: QBD4)
CO2 incubator (Sanyo, model: MCO-18AIC)
Alcohol burner (LabTek, model: LW15557-01)
Tube roller mixer (Ratek, model: BTR10P-12V)
Magnetic stirrer (IKA, model: RCT basic)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Greaney, J., Subramanian, G. N., Ye, Y. and Homer, H. (2021). Isolation and in vitro Culture of Mouse Oocytes. Bio-protocol 11(15): e4104. DOI: 10.21769/BioProtoc.4104.
- Subramanian, G. N., Greaney, J., Wei, Z., Becherel, O., Lavin, M. and Homer, H. A. (2020). Oocytes mount a noncanonical DNA damage response involving APC-Cdh1-mediated proteolysis. J Cell Biol 219(4): e201907213.
分类
发育生物学 > 繁殖
细胞生物学 > 细胞分离和培养 > 细胞分离
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link