- EN - English
- CN - 中文
A Multi-color Bicistronic Biosensor to Compare the Translation Dynamics of Different Open Reading Frames at Single-molecule Resolution in Live Cells
一种多色双顺反子生物传感器比较活细胞中不同开放阅读框在单分子分辨率下的翻译动力学
发布: 2021年07月20日第11卷第14期 DOI: 10.21769/BioProtoc.4096 浏览次数: 4051
Abstract
Here, we describe how to image and quantitate the translation dynamics of a bicistronic biosensor that we recently created to fairly compare cap-dependent and IRES-mediated translation at single-molecule resolution in live human cells. This technique employs a pair of complementary intrabodies loaded into living cells that co-translationally bind complementary epitopes in the two separate ORFs of the bicistronic biosensor. This causes the biosensor to fluoresce in different colors depending on which ORF/epitopes are translated. Using the biosensor together with high-resolution fluorescence microscopy and single-molecule tracking analysis allows for the quantitative comparison of translation dynamics between the two ORFs at a resolution of tens-of-nanometers in space and sub-seconds in time, which is not possible with more traditional GFP or luciferase reporters. Since both ORFs are on the same biosensor, they experience the same microenvironment, allowing a fair comparison of their relative translational activities. In this protocol, we describe how to get this assay up and running in cultured human cells so that translation dynamics can be studied under both normal and stressful cellular conditions. We also provide a number of useful tips and notes to help express components at appropriate levels inside cells for optimal live cell imaging.
Graphical abstract:
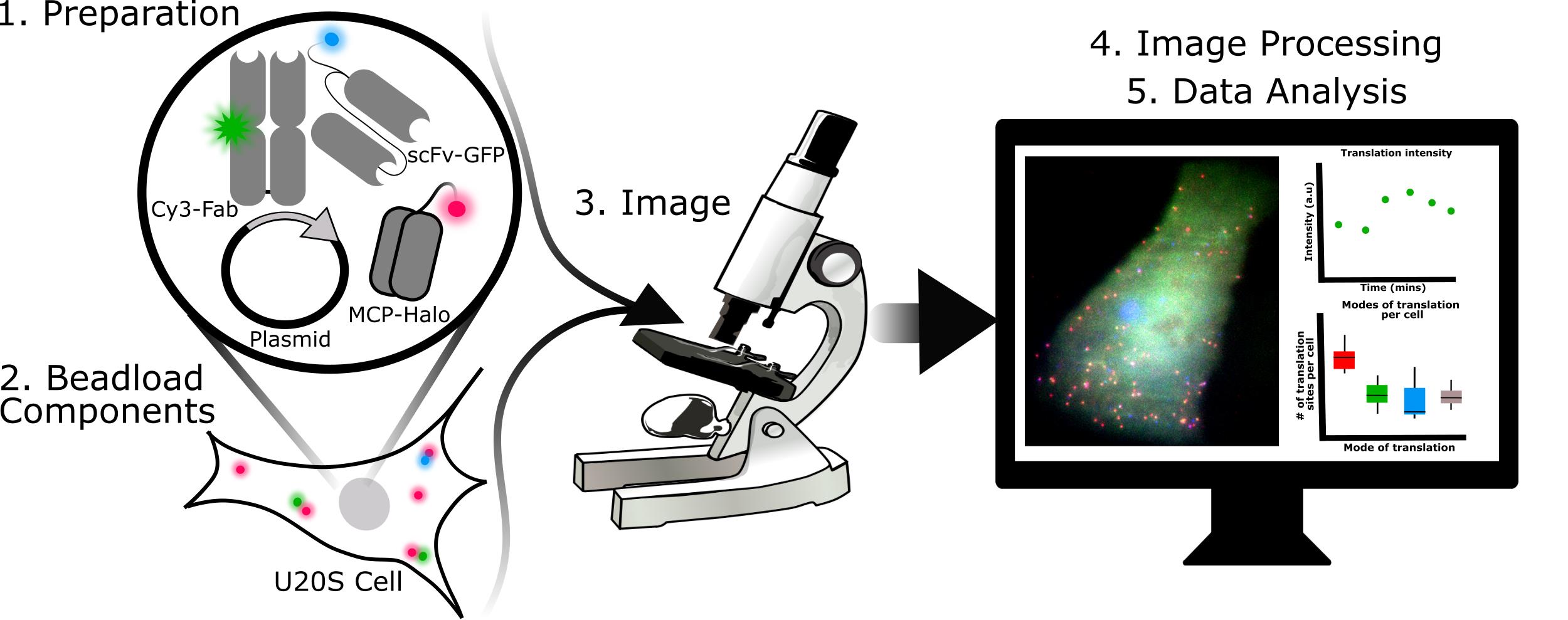
Steps required for 3-color single-molecule translation imaging and analysis.
Background
Recently, it has become possible to measure the translation dynamics of single mRNA molecules in live cells (Morisaki et al., 2016; Pichon et al., 2016; Wang et al., 2016; Wu et al., 2016; Yan et al., 2016). This technology allows for the quantitation of the heterogeneity of translation dynamics among mRNA (Morisaki and Stasevich, 2018). To image and quantitate single mRNA translation dynamics, fluorescent intrabodies such as Fab (Hayashi-Takanaka et al., 2011), scFv (Tanenbaum et al., 2014; Zhao et al., 2019), or nanobodies (Boersma et al., 2019) are required. These intrabodies bind and label repeated epitopes inserted at the N-terminus of a protein of interest. As the protein of interest is translated, the repeated epitopes emerge from the ribosome and are bound within seconds by the fluorescent intrabodies (Morisaki and Stasevich, 2018; Cialek et al., 2020). This strategy amplifies fluorescence within the translation sites at two levels: firstly, multiple fluorescent intrabodies can bind the repeated epitopes within a single nascent peptide chain at the same time; and secondly, multiple ribosomes can translate the mRNA in polysomes to produce multiple nascent peptide chains within a single translation site. These two levels of amplification produce bright fluorescent puncta that can be detected with single-molecule precision above the background using a sensitive fluorescence microscope. As ribosomes initiate, elongate, and terminate translation, the fluorescence intensity at individual translation sites fluctuates up or down, yielding insight into translation dynamics.
Over the last few years, complementary intrabodies and epitopes have been developed that make it possible to image translation in multiple colors at the same time. For example, our lab has developed anti-FLAG and anti-HA Fab that bind the classic FLAG and HA epitopes (Morisaki et al., 2016). More recently, we developed a genetically encodable scFv version of the anti-HA Fab that we call the anti-HA frankenbody (Zhao et al., 2019). Likewise, the Tanenbaum lab has developed the SunTag (SunTag epitopes + anti-SunTag scFv-GFP) and MoonTag (MoonTag epitopes + anti-MoonTag nanobody) systems (Tanenbaum et al., 2014; Boersma et al., 2019). Used together, these complementary imaging tools make it possible to compare the dynamics of different open reading frames (ORFs) at the single-molecule level within live cells.
Here, we describe how to use a 3-color bicistronic biosensor that we recently developed to compare translation kinetics at internal ribosomal entry sites (IRES) versus at the canonical 5’ cap (Figure 1a) (Koch et al., 2020). The biosensor encodes an MS2 tag to mark and track individual mRNA molecules (red) (Coulon et al., 2013; Pichon et al., 2020). When these mRNAs are expressed in cells, they light up in different colors depending on the nature of translation initiation. If translation is initiated at the cap, then FLAG epitopes are translated and bound by anti-FLAG Fab conjugated with Cy3 (green). If translation is initiated at the IRES, then SunTag epitopes are translated and bound by anti-SunTag scFv fused to eGFP (blue). This produces a variety of colorful puncta in cells that mark individual mRNA and their translation status (Figure 1a and 1c).
This basic imaging biosensor is unique because it enables quantitative comparisons between the translation dynamics of two ORFs at a spatial resolution on the tens-of-nanometers scale and temporal resolution on the sub-seconds scale. This is not possible with more traditional bulk assays that lack single-molecule resolution, including traditional luciferase and GFP reporters as well as bulk assays like western blotting. The biosensor is also versatile because a different IRES sequence can easily be inserted to study the translational dynamics of that IRES in diverse cell types and cellular conditions (Bornes Stéphanie et al., 2007; Firth and Brierley, 2012). Further, our 3-color assay can be extended to compare ORF translation dynamics on two separate mRNAs (Figure 1b). This can be achieved by simply splitting the bicistronic biosensor into two monocistronic biosensors, each encoding a probe/epitope pair and an MS2 tag. This setup is not only beneficial for comparing the translation dynamics of two different ORFs but also for fairly comparing different endogenous 5’ or 3’ UTRs or viral elements within single cells.
In this protocol, we focus mainly on the wet lab skills needed to express our biosensor in live human cells. In particular, since our biosensor fluoresces in three colors, multiple components need to be expressed within cells at appropriate levels. Here, we advocate the use of beadloading (McNeil and Warder, 1987; Hayashi-Takanaka et al., 2011) to achieve this with high efficiency and minimal effort. We describe how the various probes – including the MS2 coat protein used to label mRNA with an MS2 tag, Fab to label FLAG-tagged epitopes, and SunTag scFv to label SunTag epitopes – can be purified and loaded together in a single, simple step (Cialek et al., 2021; Morisaki et al., 2016; Koch et al., 2020). We find this approach to be very convenient, enabling the fine-tuning of probe levels, rapid testing, and combinatorial experimentation. Finally, we describe the basics of our imaging and analyses, including a specific test to verify translation, useful conditions for stressing cells while under the microscope, and basic codes to quantitate translation site intensities and distributions.
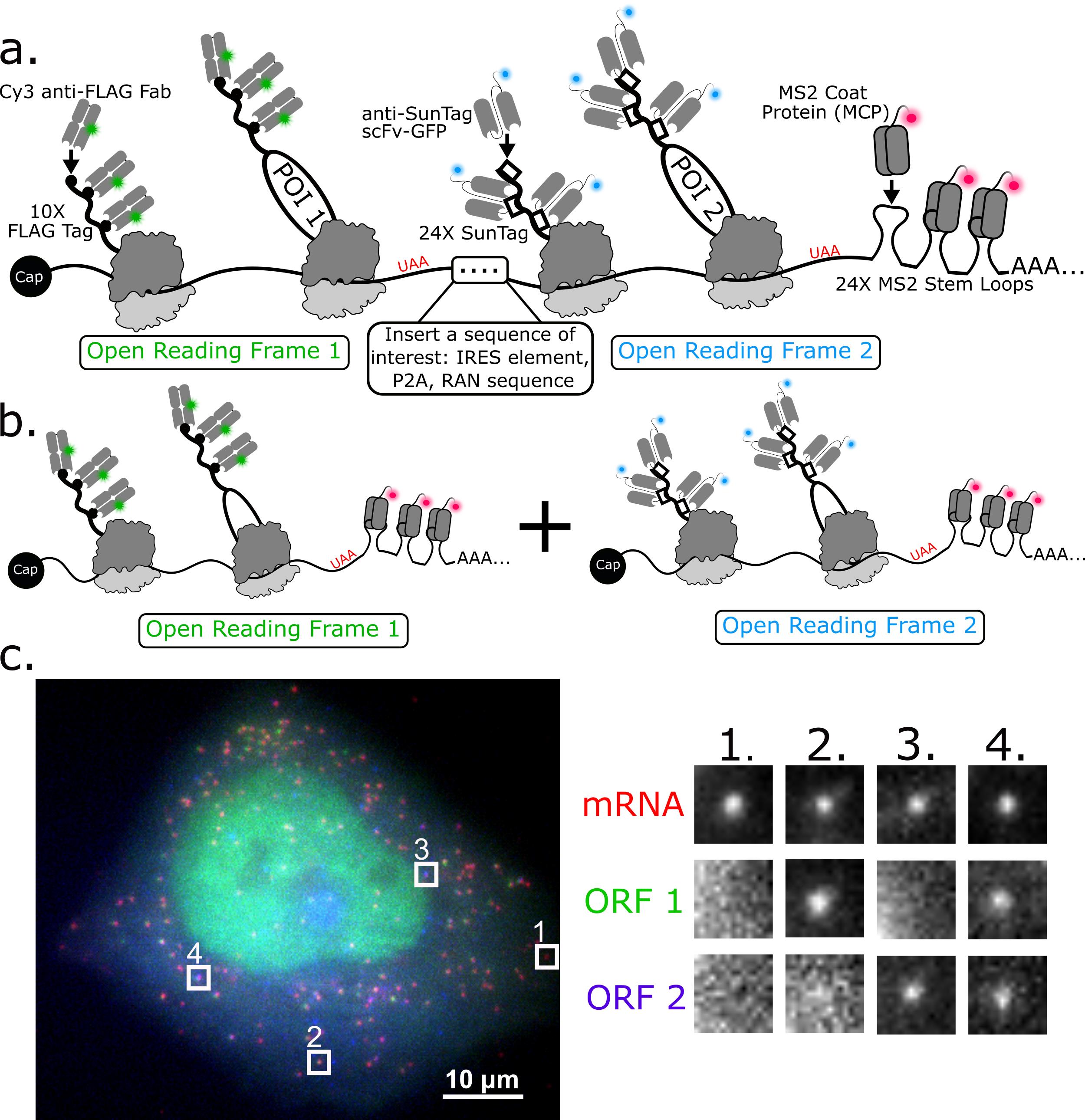
Figure 1. 3-color imaging of the translation of a single bicistronic or pair of monocistronic biosensors in live cells. a) Schematic illustrating a bicistronic 3-color single-molecule translation reporter. b) Schematic illustrating a 2-mRNA single-molecule translation reporter system. c) A 3-color bicistronic reporter (representative cell). Crops showing all types of translation are on the right panel.
Materials and Reagents
Anti-FLAG Fab generation and dye-conjugation
Pierce Fab preparation kit (Thermo Fisher Scientific, catalog number: 44985)
0.6 ml or 1.7 ml low retention tubes (Thomas Scientific, catalog numbers: 1149K01 and 1159M35PK, respectively)
PD-mini G-25 desalting column (GE Healthcare, catalog number: 95055-984)
Amicon Ultracel-50 (50 kDa-cutoff) 15-ml centrifugal filter unit (Millipore Sigma, catalog number: UFC9050)
Amicon Ultracel-10 (10 kDa-cutoff) 15-ml centrifugal filter unit (Millipore Sigma, catalog number: UFC9010)
Amicon Ultracel-10 (10 kDa-cutoff) 0.5-ml centrifugal filter unit (Millipore Sigma, catalog number: UFC5010)
FLAG antibodies (FujiFilm Wako Pure Chemical Corporation, catalog number: 012-22384 (Anti-DYKDDDDK mouse monoclonal IgG2b antibodies)
Cy3 N-hydroxysuccinimide ester mono-reactive dye pack (VWR, catalog number: 95017-373 PK)
Dimethyl sulfoxide (DMSO) (Millipore Sigma, catalog number: D8418)
Sodium bicarbonate (NaHCO3) (Millipore Sigma, catalog number: S5761-500G)
Phosphate-buffered saline (PBS) (Thermo Fisher Scientific, catalog number: AM9625)
Halo-MCP and anti-SunTag scFv-GFP purification
BL21 E. coli (DE3) pLysS competent cells (Novagen, EMD millipore, catalog number: 69451-3)
2× YT growth media (Research Products International, catalog number: X15600-5000.0)
Ampicilllin (sodium) USP grade (GoldBio, catalog number: A-301-100)
Chloramphenicol USP grade (GoldBio, catalog number: C-105-100)
Isopropyl-β-D-thiogalactoside (IPTG) (GoldBio, catalog number: I2481C100)
Phosphate-buffered saline (PBS) pH 7.4
Sodium chloride (NaCl) ACS grade
Protease inhibitor cocktail tablets (PierceTM EDTA-free Protease Inhibitor Mini Tablets, Thermo Scientific, catalog number: A32955)
4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF serine protease inhibitor) (GoldBio, catalog number: A-540-10)
Imidazole ACS reagent, ≥ 99% titration (Sigma-Aldrich)
Amicon Ultracel-30 (30 kDa-cutoff) 15-ml centrifugal filter unit (EMD Millipore, catalog number: UFC903024)
HEPES-based buffer (see Recipes)
HisTrap buffer A (see Recipes)
HisTrap buffer B (see Recipes)
Superdex buffer (see Recipes)
U-2 OS cell culture
U-2 OS cells (ATCC, catalog number: ATCC HTB-96)
DMEM (+/-), high glucose, no glutamine (See Recipes for DMEM (+) media) (Thermo Fisher Scientific, catalog number: 11960069)
Fetal bovine serum (Atlas Biologics, catalog number: F-0050-A)
Penicillin-streptomycin (Thermo Fisher Scientific, catalog number: 15140-122)
L-glutamine, 200 mM (100×) (Thermo Fisher Scientific, catalog number: 25030081)
Trypsin (Thermo Fisher Scientific, catalog number: 25300062)
100-mm cell culture dishes (VWR, catalog number: 82050-916)
Beadloading and staining Halo-MCP with JF646 ligand
106-µm glass beads (Millipore Sigma, catalog number: G4649)
Spectramesh Woven Filters Polypropylene Opening: 105-µm (Spectrum Labs, catalog number: 148496)
Plasmid DNA of interest
Cy3 anti-FLAG Fab (prepared as described in Step B8)
Purified anti-SunTag scFv-GFP (prepared as described in Step C19)
Purified Halo-MCP (prepared as described in Step C19)
Phenol-free DMEM (Thermo Fisher Scientific, catalog number: 31053036)
Janelia Fluor 646 HaloTag ligands (Promega, catalog number: GA1120)
0.6-ml low retention tubes (Thomas Scientific, catalog number: 1149K01)
Phosphate-buffered saline (Thermo Fisher Scientific, catalog number: AM9625)
Imaging
Glass-bottomed dishes, 35-mm, #1.5, 14-mm glass, uncoated (MatTek Corporation, catalog number: P35G-1.5-14-C)
Opti-MEM, Reduced Serum Medium (Thermo Fisher Scientific, catalog number: 31985070)
Puromycin (Thermo Fisher Scientific, catalog number: A1113803)
Harringtonine (Cayman Chemical Company, catalog number: 26833-85-2)
Sodium arsenite (NaAs) (Millipore Sigma, catalog number: S7400-100G)
Dithiothreitol (DTT) (Thermo Fisher, catalog number: R0861)
Equipment
UV-Vis spectrophotometer (Thermo Fisher Scientific, model: NanoDrop OneC)
Table top centrifuge (Beckman Coulter, model: Microfuge 20)
Cooled centrifuge (Thermo Fisher Scientific, model: Sorvall Legend XFR with F14 6x250LE)
Sonicator
HisTrap HP, 5-ml (Cytiva, formerly GE Healthcare catalog number: 17524801)
HiLoad 16/600 Superdex 200 pg column (Cytiva catalog number 28989335)
Fluorescence microscope (capable of thin sectioning; confocal, light-sheet, and HILO microscopes recommended) with a stage-top incubator
Software
Software for single-molecule tracking, e.g., TrackMate (Tinevez et al., 2017) or our custom Mathematica code found at:
https://github.com/Colorado-State-University-Stasevich-Lab/mRNA_Tracking_BioProtocol
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Koch, A. L., Morisaki, T. and Stasevich, T. J. (2021). A Multi-color Bicistronic Biosensor to Compare the Translation Dynamics of Different Open Reading Frames at Single-molecule Resolution in Live Cells. Bio-protocol 11(14): e4096. DOI: 10.21769/BioProtoc.4096.
分类
生物物理学 > 显微技术
分子生物学 > RNA > mRNA 转译
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link