- EN - English
- CN - 中文
Monitoring Changes in the Oxidizing Milieu in the Endoplasmic Reticulum of Mammalian Cells Using HyPerER
利用HyPerER监测哺乳动物细胞内质网氧化环境的变化
发布: 2021年07月05日第11卷第13期 DOI: 10.21769/BioProtoc.4076 浏览次数: 3884
评审: Begoña DiazAnonymous reviewer(s)

相关实验方案
高灵敏且可调控的 ATOM 荧光生物传感器:用于检测细胞中蛋白质靶点的亚细胞定位
Harsimranjit Sekhon [...] Stewart N. Loh
2025年03月20日 2118 阅读
Abstract
The production of reactive oxygen species (ROS) and endoplasmic reticulum (ER) stress are tightly linked. The generation of ROS can be both the cause and a consequence of ER stress pathways, and an increasing number of human diseases are characterized by tissue atrophy in response to ER stress and oxidative injury. For the assessment of modulators of ER luminal ROS generation and for mechanistic studies, methods to monitor changes in ER reduction-oxidation (redox) states in a time-resolved and organelle-specific manner are needed. This has been greatly facilitated by the development of genetically encoded fluorescent probes, which can be targeted to different subcellular locations by specific amino acid extensions. One of these probes is the yellow fluorescent protein-based redox biosensor, HyPer. Here, we provide a protocol for the time-resolved monitoring of the oxidizing milieu in the ER of adherent mammalian cells using the ratiometric sensor, HyPerER, which is specifically targeted to the ER lumen.
Keywords: Endoplasmic reticulum (内质网)Background
The endoplasmic reticulum (ER) plays key roles in essential functions including protein folding and maturation in the secretory pathway, lipid metabolism, hormone synthesis, and detoxification of reactive metabolites (Bock and Kohle, 2009; Chen and Cubillos-Ruiz, 2020; Erdbrugger and Frohlich, 2020; Morishita and Arvan, 2020). Research on the ER has attracted increasing interest in recent years, mainly due to the discovery of the unfolded protein response (UPR) signaling pathway, which is triggered by diverse forms of protein folding stress in the ER. The physical contact sites of the ER with other cell organelles and their involvement in cellular communication networks establish the ER as a multifaceted regulator of cell signaling. The relationship between ER stress and oxidative injury has been extensively investigated; however, the origin of ER stress-induced ROS production remains unclear (Appenzeller-Herzog, 2011), and tools to detect xenobiotics that enhance ROS in the ER are limited.
To understand the mechanisms of oxidative insults, specific tools are required to quantitate and describe ER redox conditions. Genetically encoded sensors to quantitate the oxidative status of the many redox couples present in the ER have proven useful. Changes in the amount of H2O2 in the cytoplasm can be monitored using the fluorescent probe, HyPer (Belousov et al., 2006). The HyPer sensor was constructed by inserting a circularly permutated yellow fluorescent protein (YFP) into the regulatory domain of the bacterial H2O2-sensing protein, OxyR. Importantly, HyPer was shown to selectively detect H2O2 over superoxide, peroxinitrite, nitric oxide, and oxidized glutathione in the cytosol. Upon oxidation of the cysteine corresponding to Cys199 of OxyR, the sensor protein HyPer undergoes a conformational change. HyPer has two excitation peaks at 420 nm and 500 nm, and one emission peak at 516 nm. Upon transition from the reduced to the oxidized state, the peak at 420 nm decreases and the peak at 500 nm increases, thus allowing ratiometric measurement of H2O2.
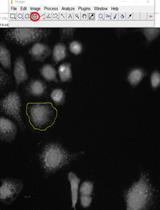
Here, we describe a detailed protocol for the real-time imaging and monitoring of the oxidizing milieu in the ER using the HyPerER sensor, which is targeted specifically to the ER lumen (Enyedi et al., 2010; Malinouski et al., 2011) by the addition of an N-terminal ER-targeting sequence and a C-terminal ER-retrieval signal (KDEL). It must be noted that the ER-targeted HyPerER, unlike cytosolic HyPer, is not specific for H2O2 but rather reflects the oxidative milieu within the ER (Mehmeti et al., 2012). The approach can be used in different cellular systems with basic understanding of live cell imaging and fluorescence microscopy (an example is shown in Figure 1), whereby data analysis is dependent on the available software. A limitation of the original HyPer sensors is their sensitivity to pH changes. To overcome this limitation, a very recent study introduced a new generation HyPer probe, HyPer7, which is pH-resistant yet remains ultra-sensitive to changes in H2O2 (Pak et al., 2020). Monitoring H2O2 specifically in the ER remains challenging, and most of the probes available to date have not been used in this very special cellular compartment.
Materials and Reagents
Materials
Pipette tips
Glass-bottomed dishes (such as MatTek, catalog number: P35G-1.5-20-C; IBIDI µ-Dish 35 mm, high Glass Bottom, catalog number: 81158; Sarstedt lumox dish 35, catalog number: 94.6077.331; Nunc Glass Bottom Dishes, catalog number: 150680)
1.5-ml tubes
50-ml conical centrifugation tubes
Reagents
HeLa cells (ATCC, catalog number: CCL-2)
Dulbecco’s Modified Eagle’s Medium (DMEM) – high glucose (Sigma-Aldrich, catalog number: D5796)
Fetal bovine serum, FBS (South America) (Biowest, catalog number: S1810)
Penicillin/streptomycin 100× (BioConcept-Amimed, catalog number: 4-01F00-H)
Trypsin-EDTA solution 10× (Sigma-Aldrich, catalog number: T4174-100ML)
OptiMEM-I (Gibco, catalog number: 51985026)
FuGENE HD (Promega, catalog number: E2311)
HEPES (PanReac AppliChem, catalog number: A1069)
CaCl2·2H2O (Merck, catalog number: 1.02382.0500)
KCl (Merck, catalog number: 1.04936.1000)
MgCl2·6H2O (Fluka, catalog number: 63064)
NaCl (PanReac AppliChem, catalog number: A2942)
DTT (PanReac AppliChem, catalog number: A1101)
H2O2 solution (Sigma-Aldrich, catalog number: 95321)
pCMV/myc/ER/GFP HyPerER (Enyedi et al., 2010) (Kind gift from Dr. Miklos Geiszt, Semmelweis University, Budapest, Hungary)
Stimulants and inhibitors (experiment-dependent), e.g., thapsigargin (EMD Millipore, catalog number: 586005)
HEPES Imaging Buffer (1 L) (see Recipes)
Equipment
Pipettes
Casy cell counter (Omni Life Science) or hemocytometer
Heat block for 50-ml conical tubes
Inverted microscope; we use an Olympus Fluoview3000 laser scanning microscope
60× Objective UPLSAPO60XS2 Universal Plan Super Apochromat silicone immersion objective N.A. 1.3 (N5203000)
For excitation, we use an Olympus FVL-LAS405-LX50 Laser 405 nm and FVL-LAS488-LS20 Laser 488 nm (somewhat below the excitation peaks at 420 nm and 500 nm, respectively)
Note: Monitoring of HyPer probes does not require confocality! A fluorescence microscope with suitable filter sets for excitation and emission is sufficient.
Excitation maximum of HyPer: 500 nm
Emission maximum of HyPer: 516 nm
Climate control unit; we use the Olympus CellVivo incubator system for IX83 (E0439957)
Note: Live cell experiments should be performed under optimal environmental conditions; the minimal requirement is a temperature controller to maintain the optimal temperature of 37°C; for long-term experiments, e.g., longer than 30 min, an additional CO2 supply will be needed.
Software
FV300 (Olympus)
Excel (Microsoft)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Birk, J., Lizak, B., Appenzeller-Herzog, C. and Odermatt, A. (2021). Monitoring Changes in the Oxidizing Milieu in the Endoplasmic Reticulum of Mammalian Cells Using HyPerER. Bio-protocol 11(13): e4076. DOI: 10.21769/BioProtoc.4076.
分类
癌症生物学 > 细胞能量学
生物化学 > 蛋白质 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link