- EN - English
- CN - 中文
An Improved Method for Individual Tracking of Voluntary Wheel Running in Pair-housed Juvenile Mice
一种对饲养的幼鼠自主跑轮运动个体跟踪的改进方法
发布: 2021年07月05日第11卷第13期 DOI: 10.21769/BioProtoc.4071 浏览次数: 4719
评审: Soyun KimDhruv Rajanikant PatelAnonymous reviewer(s)
Abstract
Rodent cages equipped with access to a voluntary running wheel are commonly used to study the effects of aerobic physical activity on physiology and behavior. Notable discoveries in exercise neurobiology, including the key role of brain-derived neurotrophic factor (BDNF) in neural plasticity and cognition, have been made using rodents housed with voluntary running wheels. A major advantage of using home-cage running wheels over treadmills is the elimination of stress potentially associated with forced running. In addition, voluntary wheel running may simulate a more natural running pattern in laboratory mice. Singly housing mice with voluntary running wheels is traditionally employed to obtain exact quantitation of the distance ran; however, social isolation stress is often ignored to obtain precise running distances. Moreover, voluntary exercise studies in adolescent mice must consider the neurodevelopmental implications of isolation stress. In this protocol, we wean 21-day-old mouse pups directly into running wheel-equipped cages and pair-house them to reduce the impact of social isolation and other developmentally specific factors that could adversely affect their behavior or development. Individual running distances are obtained from each mouse in the cage using a radio-frequency identification (RFID) ear tag and a hidden antenna placed directly under the running wheel. We have demonstrated that voluntary running during a specific juvenile-adolescent developmental period can improve hippocampal memory when tested during adolescence (Ivy et al., 2020). Individual exercise tracking of group-housed mice can enable future studies to precisely correlate the amount of exercise with readouts such as cell-specific gene expression, epigenetic mechanisms, serum biomarkers, and behavior, in an intra-individual manner.
Graphic abstract:
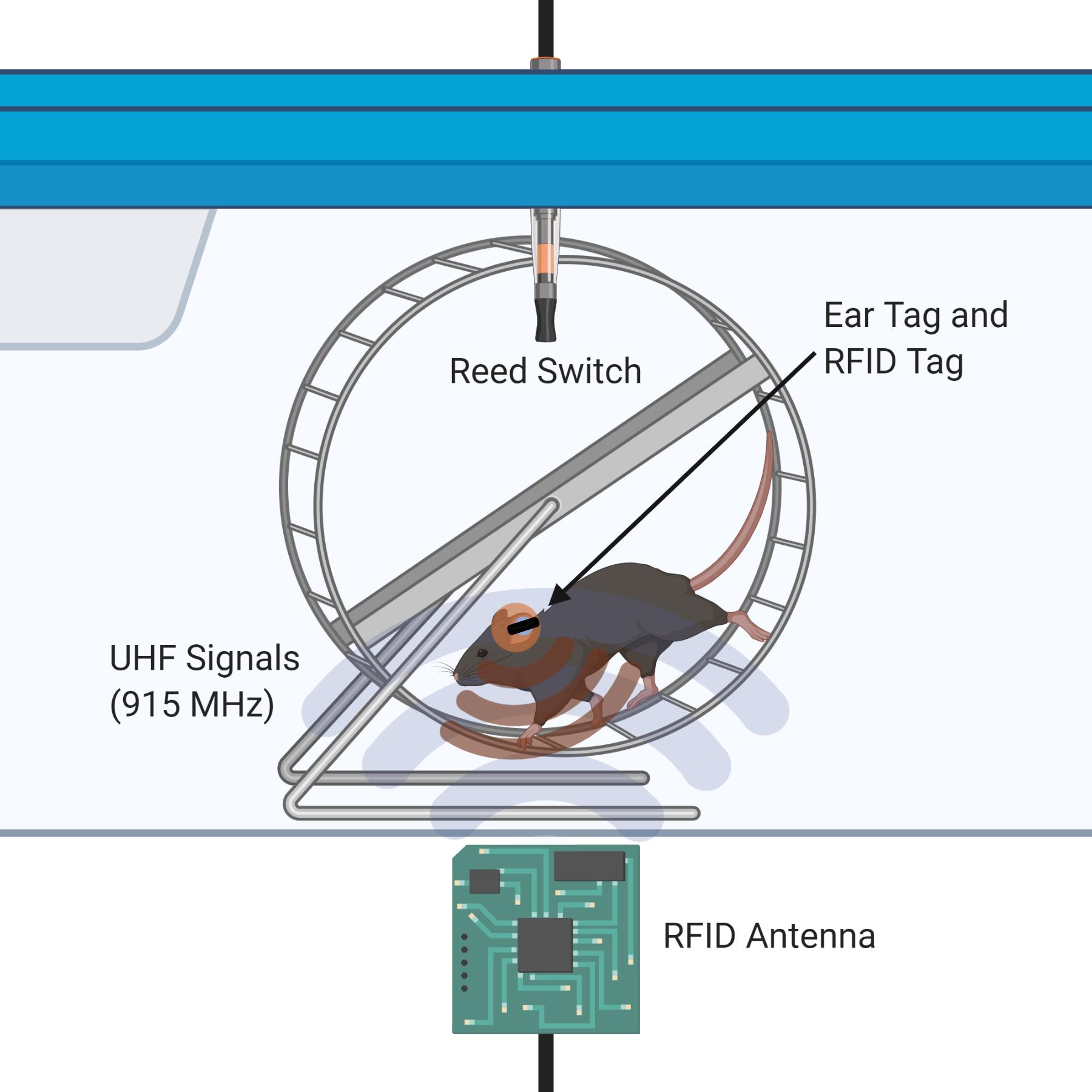
Figure 1. Illustration of the dual RFID and Vital View system for individual mouse running in a pair-housed cage
Background
The use of voluntary running wheels in laboratory rodent cages is a common approach for investigating the impact of exercise on brain function and neurodegeneration (Liu et al., 2019). Employing a voluntary exercise study design eliminates the need for a stressful stimulus, such as a foot shock or investigator handling, to encourage the animal to exercise. Studies utilizing in-cage voluntary running wheels have traditionally housed rodents individually to generate accurate running distances and capture individual variations in exercise amounts (Goh and Ladiges, 2015). Individual running data can then be correlated with other intra-individual datasets. For example, total distance run over a 2-6 hour period positively correlates with brain-derived neurotrophic factor (BDNF) gene expression in the hippocampus (Oliff et al., 1998). However, a disadvantage of individual housing is the potential for stress induced by social isolation. Since mice are social animals, their innate social behaviors (whether in wild or captive environments) develop most naturally in social arrangements similar to those found in wild colonies (Reimer and Petras, 1967). The controlled laboratory environments required for accurate data collection frequently result in housing conditions that challenge the formation of natural social structures. The lack of a social environment when mice are individually housed must be considered not only for rodent welfare but also for its impact on the quality of data produced and the interpretation of findings (Kappel et al., 2017; Arakawa, 2018).
Prior research has demonstrated that individual housing of mice can impact brain function in a number of ways. It can alter hypothalamic-pituitary-adrenocortical (HPA) axis function, specifically glucocorticoid regulation and feedback (Hawkley et al., 2012). Individual housing can also confound performance on various behavioral tests, including those assessing anxiety (Koike et al., 2009) and learning and memory (Okada et al., 2015). Finally, single housing can lower the expression of neuroplasticity-related genes in the hippocampus and prefrontal cortex (Ieraci et al., 2016). Social isolation stress in singly housed rodents is therefore an important variable to consider when interpreting studies that assess the neurobiological effects of voluntary exercise.
On the other hand, group housing of mice can invoke male-on-male aggression. This usually emerges in rodents after the onset of puberty and tends not to be present during juvenile and adolescent developmental stages (Terranova et al., 1998). Female mice have a lower tendency to exhibit this aggression, even post-puberty (Hayes, 2000). Social isolation in juvenile rodents adversely impacts myelination in the medial prefrontal cortex (Makinodan et al., 2012), whereas social play in juveniles can enhance neural plasticity in this region (Himmler et al., 2013). Moreover, juvenile mice use the body temperature of their cage mates for thermoregulation (Batchelder et al., 1983). This underscores the importance of paired or grouped housing for maintaining basal body temperature, particularly in the setting of shifting metabolic demands with exercise. Therefore, in studies linking juvenile voluntary wheel running with neural function and behavior, a group housing-based approach may be preferred to eliminate isolation stress.
Individual home-cage rodent tracking has been accomplished through the use of video (Krynitsky et al., 2020) (Poffe et al., 2018) (Wang et al., 2018), subcutaneously implanted RFID microchips (Peleh et al., 2019) (Frahm et al., 2018), a combination of the two, or passive infrared sensors (Matikainen-Ankney, Garmendia-Cedillos et al., 2019). Current protocols utilizing subcutaneously implanted RFID microchips require prolonged restraint and anesthesia, which may produce undesired stress and pain. Unified Information Devices details a protocol that describes safely implanting a mouse RFID microchip (UID UC-1485) without anesthesia on postnatal day 12. This approach is used primarily for mouse identification at a single point in time, such as for taking rapid mouse inventory within a group-housed cage, but its use has not been demonstrated for live, continuous tracking. Another factor is the size of the RFID chip, which must be large enough to be detected by the antenna. For juvenile mice, typically weighing 6-9 grams, this size requirement prohibits administration without the use of anesthesia. In video tracking, wire cage tops obstruct continuous top-down video recording but are required for many home-cage running wheel systems. Finally, existing home-cage RFID activity-tracking systems currently on the market use a matrix of RFID antennae arranged throughout the base of the cage or in strategic locations to monitor baseline ambulatory activity (Voikar and Gaburro, 2020). However, if the objective of the experiment is to track voluntary wheel-running, only one antenna with a read range precisely limited to the area inside the wheel is required.
Our apparatus uses strong, low-profile RFID ear tags and one RFID antenna at the base of each cage to present a minimally invasive alternative to existing video-based and implantation-based tracking systems (Figure 1). The major advantage of our protocol is the ability to individually track running wheel activity of pair-housed juvenile mice starting at the age of weaning (postnatal day 21). We reduce isolation stress and issues with thermoregulation by pair-housing the mice and providing nesting material. Indeed, this model can be scaled up to >2 mice per cage if preferred. This procedure has been tested in cages containing mice of the same sex, but we did not investigate mixed-sex population effects on individual running activity. Our experimental design allows for the comparison of individual running distances between female and male pairs of mice within the same cage. Although there may be territorial issues once social hierarchy is established in cages housing two or more male mice, a precise quantity of exercise from each individual mouse in a group-housed cage can be correlated with any desired experimental readout.
A minor limitation of this apparatus is that in our hands, about 5-10% of running activity is unable to be reconciled; however, we believe that our 90-95% accuracy of allocating individual running data in group-housed environments is an acceptable yield. Although this method does not encounter the same issues with accuracy-diminishing antenna cross-talk as observed in RFID antenna matrices (Voikar and Gaburro, 2020), the metal wheel in very close proximity to the antenna may have the same interfering effect (thus necessitating a large ear tag). Our design effectively limits the read range of the antenna to the wheel area only.
Investigating the effects of voluntary physical activity has numerous implications for increasing our understanding of its benefits toward brain function and behavior. Determining individualized amounts of exercise for specific outcomes or targets requires accurate monitoring of running distances, which can now be performed as described in this protocol. Our approach can track individual mice by recognizing the unique RFID tags of pair-housed mice coupled with time-stamped data obtained from a magnetic sensor on a home-cage running wheel. Importantly, the procedure can be scaled up to tracking running distances of multiple mice in a cage sharing access to one running wheel. Our method brings RFID applications to juvenile mouse exercise studies, while minimizing stress from isolation and restraint, eliminating the need for anesthesia, and producing highly precise and individualized running data.
Materials and Reagents
UHF RFID Tag for Metal Product Management (Murata Manufacturing, catalog number: LXTBKZMCMG-010)
CommandTM Small Poster Strips (3M, catalog number: 17024ES)
Pegboard, 5/32” thickness, holes spaced 1” apart
LOCTITE® SUPER GLUE PRECISION PEN (Henkel, catalog number: 2066118)
Netting material (Industrial Netting, catalog number: NG3060-164)
Ear Tag, Mouse, Light Blue, 1-100 (Stoelting, catalog number: 56782)
Ear Tag, Backing only, Pk/100 (Stoelting, catalog number: 56792)
Etching Engraver Pen (Porsin, catalog number: LX1323)
Teklad 1/8” Corncob Bedding (Envigo, catalog number: 7092A)
Cotton Nestlets (Ancare nestlets)
Animals: Jackson mice WT C57BL/6J (Jackson Laboratories, catalog number: 000664)
Mice were progeny of C57BL/6J dams obtained from Jackson Laboratories, and were bred, born, and reared in our vivarium. Mice had free access to food and water, and the lights were maintained on a 12-hour light/dark cycle. Upon weaning on postnatal day (P) 21, mice were pair-housed in standard cages with free access to an in-cage stainless-steel running wheel equipped with a plastic net fitted around the rim for the safety of the juvenile mice. All mice are typically 6-9 g at the time of weaning. Tracking was conducted continuously 24 hours a day for three weeks from P21 to P41. All experiments were conducted according to U.S. National Institutes of Health guidelines for animal care and use and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Teklad Global Soy Protein-Free Extruded Rodent Diet (Envigo, catalog number: 2020X)
Equipment
RFID Passive Antenna (Abracon LLC: ARRAN5-915.000MHz)
L-Size Mouse Cage Body, 36.5 × 20.7 × 14.0 cm, Polycarbonate (Tecniplast Group: 1284L001; sourced from Starr Life Sciences)
Autoclavable Filter Top (Tecniplast Group: 1284L-400SU; sourced from Starr Life Sciences)
4.5" Running wheel with reed switch (Bio-Lynx scientific equipment, Inc.: 610-0003-00; sourced from Starr Life Sciences)
MMCX (J)-LL100HF-RPTNC (M) 60'' Cables (Federal Custom Cable, LLC: SCA1086-60)
Ear Tag, Applicator (Stoelting Co: 56791)
Keonn AdvanReader-160 UHF RFID Reader (4-Port) (Keonn Technologies: ADRD-M4-ESMA-160.01)
AdvanMux-8 UHF RFID Multiplexer (8-Port) (Keonn Technologies: ADMX-8-e-110.04)
CAT5 Ethernet Cable
USB 3.0-to-Gigabit Ethernet Adapter (Best Buy Co., Inc., Insignia: NS-PU98635-C)
Power Over Ethernet Injector (Phihong: PSA16U-480(POE); sourced from Keonn Technologies)
SMA Male to SMA Male 24” Cable (Bracke Manufacturing, LLC: BM92046.24)
Data Port 24 channel box (Starr Life Sciences)
Data Port USB Cable (Starr Life Sciences)
Electric Drill
Table Saw
5/32”, 3/16” Drill Bits
Two Laptops (one for each software program) with the following requirements:
Minimum Requirements for VitalView®: Windows PC (Windows XP or newer), 2 GHz Processor, 2 GB Ram, 800 MG Free Hard Disk space, USB port
Minimum Requirements AdvanNetTM Software: Firefox or Chrome browser, Internet connection, USB port
Computer (for IndividualRFIDELEMus program)
Minimum Requirements: Windows 10, 12 GB of RAM
Software
AdvanNetTM Software (Keonn Technologies, S.L., https://keonn.com/software-product/advannet/)
VitalView® Activity Data Acquisition Software (Starr Life Sciences Corp, https://www.starrlifesciences.com/product/activity-software/)
IndividualRFIDELEMus program (https://github.com/anthonyraus/IndividualRFIDELEMus/releases)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Valientes, D. A., Raus, A. M. and Ivy, A. S. (2021). An Improved Method for Individual Tracking of Voluntary Wheel Running in Pair-housed Juvenile Mice. Bio-protocol 11(13): e4071. DOI: 10.21769/BioProtoc.4071.
分类
神经科学 > 感觉和运动系统 > 动物模型
发育生物学 > 形态建成 > 能动性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link