- EN - English
- CN - 中文
Differential Analysis of N-glycopeptide Abundance and N-glycosylation Site Occupancy for Studying Protein N-glycosylation Dysregulation in Human Disease
用于研究人类疾病中蛋白质N-糖基化失调的N-糖肽丰度和N-糖基化位点的差异分析
发布: 2021年06月20日第11卷第12期 DOI: 10.21769/BioProtoc.4059 浏览次数: 4326
评审: Oneil Girish BhalalaPia GiovannelliAmberley D. Stephens

相关实验方案
高灵敏且可调控的 ATOM 荧光生物传感器:用于检测细胞中蛋白质靶点的亚细胞定位
Harsimranjit Sekhon [...] Stewart N. Loh
2025年03月20日 2259 阅读
Abstract
Protein N-glycosylation plays a vital role in diverse cellular processes, and dysregulated N-glycosylation is implicated in a variety of human diseases including neurodegenerative disorders and cancer. With recent advances in high-resolution mass spectrometry-based glycoproteomics technologies enabling large-scale N-glycoproteome profiling of disease and control samples, analysis of the large datasets has become a challenge. Here, we provide a protocol for the systems-level analysis of in vivo N-glycosylation sites on N-glycosylated proteins and their changes in human disease, such as Alzheimer's disease. The protocol includes quantitation and differential analysis of N-glycopeptide abundance, in addition to integrative N-glycoproteome and proteome data analyses, to determine disease-associated changes in N-glycosylation site occupancy and identify differentially N-glycosylated proteins in human disease versus control samples. This protocol can be modified and applied to study proteome-wide N-glycosylation alterations in response to different cellular stresses or pathophysiological states in other organisms or model systems.
Keywords: Protein N-glycosylation (蛋白质N -糖基化修饰)Background
Protein N-glycosylation – the attachment of glycans to asparagine residues – is a major posttranslational modification for regulating many key biological processes from membrane trafficking and protein degradation to immune response and cell-cell communication (Moremen et al., 2012). The importance of proper N-glycosylation in human health is underscored by the finding that mutations in the protein N-glycosylation machinery components cause congenital disorders of glycosylation with multi-system abnormalities (Freeze et al., 2015). Furthermore, increasing evidence links aberrant protein N-glycosylation to various human diseases including cancer, diabetes, and neurodegenerative diseases (Schedin-Weiss et al., 2014; Pinho and Reis, 2015; Reily et al., 2019).
A mechanistic understanding of the roles of protein N-glycosylation in biology and pathophysiology requires the system-wide elucidation of in vivo N-glycosylation sites (N-glycosites) on N-glycosylated proteins (N-glycoproteins) in health and disease. Protein N-glycosylation sites typically have a canonical sequon N-X-S|T, where X represents any amino acid except proline. However, whether a sequon-containing site can be N-glycosylated in vivo is dependent on protein folding and localization, site availability, and accessibility to N-glycosylation enzymes (Moremen et al., 2012; Cherepanova et al., 2016); thus, the in vivo N-glycosylation sites on glycoproteins have to be determined experimentally. Furthermore, since stress and pathophysiological conditions can alter N-glycosylation site occupancy and expose the cryptic N-glycosites (i.e., normally unutilized N-glycosylation sequons) for N-glycosylation (Pinho and Reis, 2015; Cherepanova et al., 2016), it is important to determine disease-associated, site-specific changes in protein N-glycosylation site occupancy to gain mechanistic insights into N-glycosylation dysregulation and its roles in disease pathogenesis.
High-resolution mass spectrometry-based glycoproteomics technologies provide powerful tools for unbiased, large-scale, site-specific N-glycoproteome profiling analyses of complex biological samples. For example, in our recently published study (Zhang et al., 2020), we used an N-glycoproteomics workflow consisting of SDS-mediated protein extraction and filter-aided sample preparation (FASP) (Wisniewski, 2017), zwitterionic chromatography-hydrophilic interaction chromatography (ZIC-HILIC)-based glycopeptide enrichment (Ma et al., 2015), 18O-labeling of in vivo N-glycosylation sites (Kuster and Mann, 1999), and liquid chromatography-tandem mass spectrometry (LC-MS/MS) to characterize protein N-glycosylation in human Alzheimer's disease (AD) and control brains. We identified 4730 18O-labeled N-glycosite-containing peptides (hereafter referred to as N-glycopeptides) and mapped 2294 in vivo N-glycosylation sites on 1132 brain N-glycoproteins (Zhang et al., 2020).
With advanced N-glycoproteomics technologies enabling simultaneous, quantitative measurement of abundance profiles for thousands of N-glycopeptides and in vivo N-glycosylation sites, analysis of such large datasets has become a challenge. In our recent study (Zhang et al., 2020), we performed differential analysis of N-glycopeptide abundance and identified 118 N-glycopeptides with >1.3-fold change in N-glycopeptide abundance in AD as compared with control cases. By integrated analysis of our N-glycoproteome and proteome profiling data from the same brain samples (Zhang et al., 2018), we identified 77 N-glycosites on 60 N-glycoproteins with >1.3-fold changes in N-glycosylation site occupancy in AD versus control brains (Zhang et al., 2020). Furthermore, we performed qualitative assessment of whether an N-glycosite was exclusively occupied in either AD or control brains and identified 89 N-glycosites on 76 glycoproteins with a gain of N-glycosylation in AD and 12 N-glycosites on 11 glycoproteins with a complete loss of N-glycosylation in AD. In total, we identified 137 differentially N-glycosylated proteins in AD versus control brains, including 92 hyperglycosylated proteins containing N-glycosites with increased N-glycosylation site occupancy and/or a gain of N-glycosylation in AD, 39 hypoglycosylated proteins containing N-glycosites with decreased N-glycosylation site occupancy or a loss of N-glycosylation in AD, and 6 aberrantly glycosylated proteins containing both hyperglycosylated and hypoglycosylated N-glycosites. These analyses have identified disease signatures of altered N-glycopeptides, N-glycoproteins, and N-glycosylation site occupancy in AD and suggest new targets for AD biomarker development (Zhang et al., 2020).
Here, we provide a protocol that describes how to perform mass spectrometry-based, systems-level analysis of in vivo N-glycosylation sites on N-glycosylated proteins and their changes in human disease (Figure 1). The protocol also includes quantitation and differential analysis of N-glycopeptide abundance, in addition to integrative N-glycoproteome and proteome data analyses, to determine disease-associated changes in N-glycosylation site occupancy and identify differentially N-glycosylated proteins in human disease versus control samples. For more details on the use and execution of this protocol, please refer to our research article (Zhang et al., 2020). Similar strategies as described in this protocol should be broadly applicable to study proteome-wide N-glycosylation alterations in response to different cellular stresses or pathophysiological conditions in other organisms or model systems.
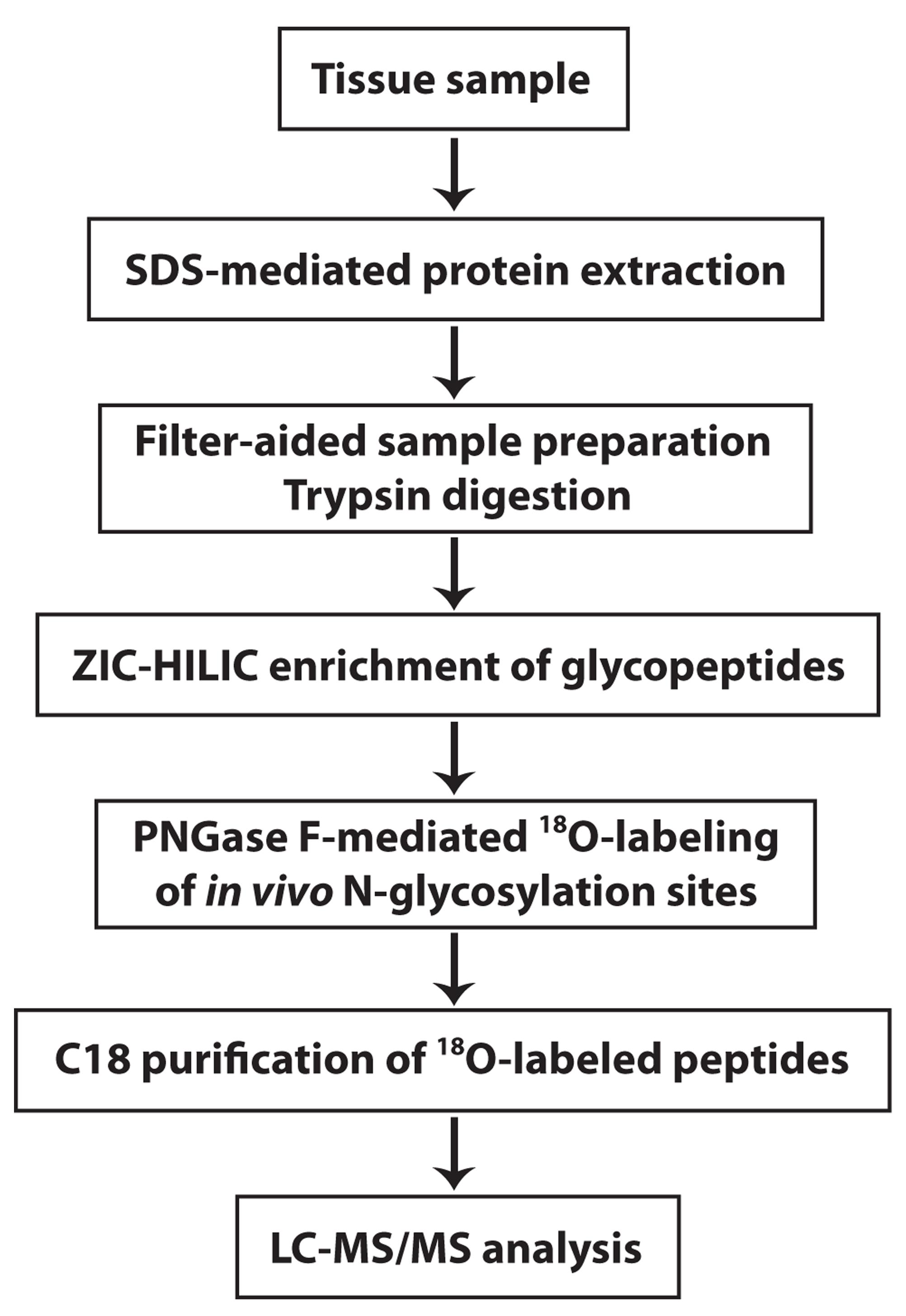
Figure 1. Overview of the experimental workflow. The experimental procedures for these steps are described in this protocol.
Materials and Reagents
Microcon 30-kDa centrifugal filter device (Merck, MRCF0R030)
Syringe, 3 ml (BD syringe)
VWR universal low-retention pipet tips, 200 μl (VWR, catalog number: 76322-150)
Bovine α2-HS-glycoprotein (fetuin) (Sigma, Millipore, catalog number: F3004-25MG)
ZIC-HILIC resin (particle size 10 μm, pore size 200 Å; Merck SeQuant, UmeÅ, Sweden)
H218O (Sigma-Aldrich, catalog number: 487090)
PNGase F (New England Biolabs, catalog number: P0704S)
Urea (Sigma, Millipore, catalog number: U1250)
Sodium dodecyl sulfate (Sigma, Millipore, catalog number: 71725)
DL-dithiothreitol (Sigma, Millipore, catalog number: D0632)
Iodoacetamide (Thermo Scientific, catalog number: A39271)
Ammonium bicarbonate (Sigma, Millipore, catalog number: 09830)
Formic acid (Sigma, Millipore, catalog number: 5330020050)
Sequencing grade modified trypsin (Promega, catalog number: V5111)
Equipment
Benchmark heat block (Benchmark Scientific)
Eppendorf 5424 microcentrifuge (Eppendorf)
Mortar and pestle, 150 ml capacity, porcelain (Grainger)
NanoDrop spectrophotometer and Quartz cuvettes (Thermo Fisher Scientific)
3MTM EmporeTM C8 extraction disk (Thermo Fisher Scientific)
PierceTM C18 tips, 100 μl bed (Thermo Fisher Scientific, catalog number: 87784)
Nano-LC UltiMate 3000 high-performance liquid chromatography system (Thermo Fisher Scientific)
LTQ-Orbitrap Elite mass spectrometer (Thermo Fisher Scientific)
EASY-Spray PepMap C18 column (length, 50 cm; particle size, 2 μm; pore size, 100 Å; Thermo Fisher Scientific)
Savant SpeedVac SC210A Plus centrifuge (Thermo Fisher Scientific)
Precision general purpose water bath (Thermo Fisher Scientific)
Software
Proteome Discoverer version 1.4 or higher (Thermo Fisher Scientific, www.thermofisher.com)
Microsoft Excel (https://www.microsoft.com)
GraphPad Prism 7 (GraphPad, https://www.graphpad.com/scientific-software/prism/)
MetaCore bioinformatics software (https://portal.genego.com)
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Zhang, Q., Ma, C., Li, L. and Chin, L. (2021). Differential Analysis of N-glycopeptide Abundance and N-glycosylation Site Occupancy for Studying Protein N-glycosylation Dysregulation in Human Disease . Bio-protocol 11(12): e4059. DOI: 10.21769/BioProtoc.4059.
- Zhang, Q., Ma, C., Chin, L.S. and Li, L. (2020). Integrative glycoproteomics reveals protein N-glycosylation aberrations and glycoproteomic network alterations in Alzheimer's disease. Sci Adv 6(40).
分类
系统生物学 > 蛋白质组学
神经科学 > 细胞机理 > 胞内信号传导
分子生物学 > 蛋白质 > 检测
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link