- EN - English
- CN - 中文
Enrichment of Vascular Fragments from Mouse Embryonic Brains for Endothelial Cell Analysis
用于内皮细胞分析小鼠胚胎脑血管片段富集
发布: 2021年06月20日第11卷第12期 DOI: 10.21769/BioProtoc.4058 浏览次数: 3413
评审: Pilar Villacampa AlcubierreAnonymous reviewer(s)
Abstract
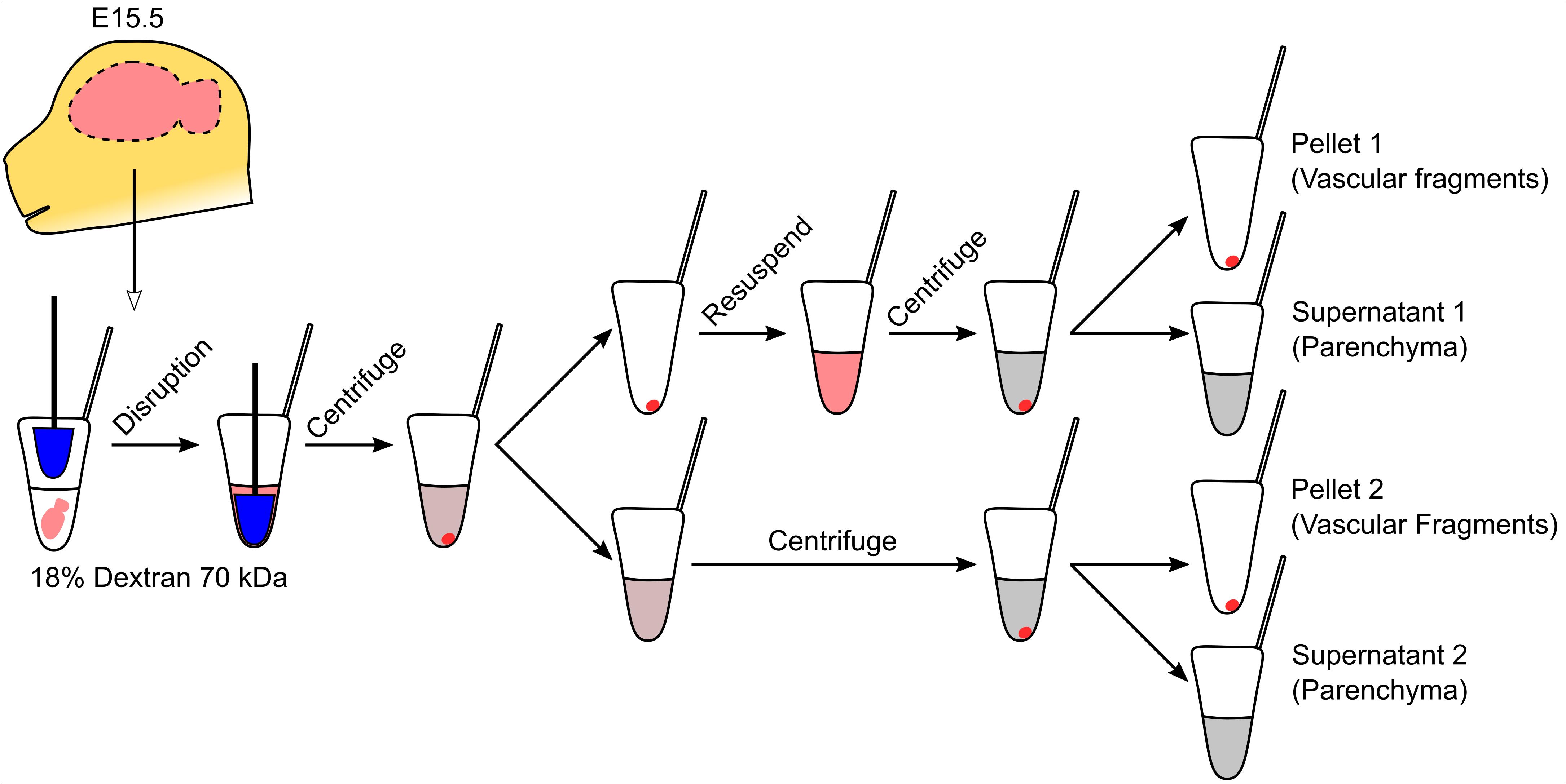
Endothelial cells in the brain interact with other cell types, forming the blood-brain barrier. This barrier controls the movement of solutes into and out of the brain, regulating pathophysiological processes and drug delivery to the brain. Common isolation methods used to study these cells during embryonic development involve enzymatic treatment and cell sorting using specific markers. This process modifies the cell state and produces minute amounts of sample. Here, we describe a protocol for the enrichment of vascular cells from embryonic brains based on dextran separation. In this method, the brain is lightly disrupted with a pestle and then resuspended in a dextran solution. Low-speed centrifugation permits the separation of the parenchymal and vascular fractions. Further centrifugation steps improve fractionation. This method is simple and fast and produces enough sample for biochemical assays.
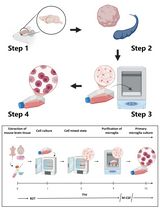
Graphic abstract:
Purification of vascular fragments from an embryonic brain
Background
Endothelial cells in the brain form a specialized barrier that limits and controls the passage of large and small molecules from the bloodstream into the brain (Zlokovic, 2008). In their natural niche, brain endothelial cells interact closely with several cell types, including pericytes, glia, and immune cells, which are integral to brain homeostasis (Armulik et al., 2005; Gavins et al., 2007). The study of brain endothelial cell biology has benefited greatly from the use of procedures to isolate either endothelium or vascular complexes, which include endothelial cells, pericytes, and glial contacts (radial glia, oligodendrocytes, and astrocyte endfeet). The former is normally achieved by fluorescence-activated cell sorting (FACS) isolation (Daneman et al., 2010), whereas the latter is done by centrifugation in a dextran solution (Yousif et al., 2007). FACS-based isolation can alter the properties of endothelial cells and is expensive and inefficient, leading to the isolation of very few cells that may not reflect their native state. These issues are more evident when obtaining samples from embryonic brains, where the sample is extremely limited, and fewer than 5000 endothelial cells are typically obtained from one brain. This number is adequate for transcriptional analyses using specialized kits (Santander et al., 2020) but is insufficient for biochemical assays. Conversely, dextran-based isolation of vascular complexes has not been applied to embryonic brains because the standard protocol requires a much bigger sample obtained from single mouse embryos. The ability to obtain enough sample for biochemical assays from single embryonic brains is necessary for the study of developmental vascular diseases, such as the Fowler syndrome (Santander et al., 2020). Here, to overcome these sample size issues, we describe the adaptation of the paradigm of dextran-based isolation of vascular fragments to individual mouse embryo brains. We demonstrate the utility of vascular fragment isolation to measure cholesterol content in embryonic brain blood vessels, an assay not feasible using FACS-isolated endothelial cells.
Materials and Reagents
15 ml conic bottom tube
DuraSeal laboratory film (Sigma, catalog number: D3172)
Polypropylene 1.5 ml tubes
Assay plate, 96-well, black
Assay plate, 96-well, clear
PVDF membrane (Thermo, catalog number: 88520)
Culture plate
Female and male mice
PBS 10× (Apex, catalog number: 20-134)
Dextran (70 kDa Highly hygroscopic, should be maintained in an inert atmosphere) (TCI, catalog number: D1449)
IGEPAL (Sigma, catalog number: I8896)
Sodium dodecyl sulfate (Sigma, catalog number: L3771)
Protease inhibitor cocktail (Thermo, catalog number: 78429)
MilliQ water
BCA Protein Assay kit (Thermo, catalog number: 23225)
Methanol (Merck, catalog number: 1060091000)
Chloroform (Merck, catalog number: 1070242500)
Amplex Red Cholesterol Assay Kit (Thermo, catalog number: A12216)
Protein loading buffer 4× (Bio-Rad, catalog number: 1610747)
2-mercaptoethanol (Sigma, catalog number: M6250)
Buffer Tris-Glycine 10× (Apex, catalog number: 18-238B)
Pre-cast 10% polyacrylamide gel (Bio-Rad, catalog number: 4561033)
Powdered skim milk
TBST 10× (Apex, catalog number: 18-235B)
Rabbit anti-ERG (Abcam, catalog number: ab92513)
Rabbit anti-PECAM1 (Santa Cruz, catalog number: sc-1506)
Rabbit anti-PAX6 (Millipore, catalog number: AB2237)
Rabbit anti-TBR1 (Abcam, catalog number: ab31940)
Mouse anti-ACTINB (R&D Systems, catalog number: MAB8929)
Donkey anti-rabbit IGG (Thermo, catalog number: A16029)
Donkey anti-mouse IGG (Thermo, catalog number: A16011)
Chemiluminescence kit (Bio-Rad, catalog number: 1705061)
PBS 1× (see Recipes)
18% Dextran (see Recipes)
Lysis buffer (see Recipes)
Running buffer (see Recipes)
Transfer buffer (see Recipes)
TBST 1× (see Recipes)
Equipment
Dumont #5 fine forceps (Fine Science Tools, catalog number: 11251-30)
Tube pestle (Sigma, catalog number: Z359947)
CO2 euthanasia chamber
Dissection microscope
Refrigerated microcentrifuge
Heating block
Fume hood
Gel documentation system
Software
LibreOffice Calc
R
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Santander, N. and Arnold, T. D. (2021). Enrichment of Vascular Fragments from Mouse Embryonic Brains for Endothelial Cell Analysis. Bio-protocol 11(12): e4058. DOI: 10.21769/BioProtoc.4058.
分类
生物化学 > 其它化合物
神经科学 > 发育
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link