- EN - English
- CN - 中文
In vivo Optical Access to Olfactory Sensory Neurons in the Mouse Olfactory Epithelium
小鼠嗅上皮嗅觉感觉神经元的体内光学通路研究
发布: 2021年06月20日第11卷第12期 DOI: 10.21769/BioProtoc.4055 浏览次数: 4123
评审: Joseph ZakWei WangAnonymous reviewer(s)
Abstract
In neuroscience, it is fundamental to understand how sensory stimuli are translated into neural activity at the entry point of sensory systems. In the olfactory system, odorants inhaled into the nasal cavity are detected by ~1,000 types of odorant receptors (ORs) that are expressed by olfactory sensory neurons (OSNs). Since each OSN expresses only one type of odorant receptor, the odor-evoked responses reflect the interaction between odorants and the expressed OR. The responses of OSN somata are often measured by calcium imaging and electrophysiological techniques; however, previous techniques require tissue dissection or cell dissociation, rendering it difficult to investigate physiological responses. Here, we describe a protocol that allows us to observe odor-evoked responses of individual OSN somata in the mouse olfactory epithelium in vivo. Two-photon excitation through the thinned skull enables highly-sensitive calcium imaging using a genetically encoded calcium indicator, GCaMP. Recording of odor-evoked responses in OSN somata in freely breathing mice will be fundamental to understanding how odor information is processed at the periphery and higher circuits in the brain.
Keywords: Olfactory system (嗅觉系统)Background
Animals recognize their environmental cues using sensory systems. The mammalian olfactory system is able to detect and discriminate a large repertoire of odorants. Odorants inhaled into the nasal cavity are detected by ~1,000 types of odorant receptors (ORs) expressed by olfactory sensory neurons (OSNs) in the olfactory epithelium (OE) of mice. Since each OSN expresses only one type of OR, the odor-evoked responses reflect the interaction between odorants and the expressed OR. To understand how odor information is translated into neural activity at the entry point of the olfactory system, it is important to study OSN responses in the olfactory epithelium in vivo.
The responses of OSN somata are often measured by calcium imaging and electrophysiological techniques (Maue and Dionne, 1987; Cygnar et al., 2010; Jarriault and Grosmaitre, 2015; Zhang, 2018); however, previous techniques require tissue dissection or cell dissociation, rendering it difficult to investigate physiological responses. Electroolfactograms can be used in vivo, but they cannot distinguish single-cell activities.
Here, we describe a protocol that allowed us to observe the odor-evoked responses of individual OSN somata in the OE in vivo (Iwata et al., 2017; Inagaki et al., 2020; Zak et al., 2020). Two-photon excitation through the thinned skull enables highly-sensitive calcium imaging using a genetically encoded calcium indicator, GCaMP (Yang et al., 2010). The preparation for in vivo imaging is simple and usually completed within 1 h. This method may apply not only to calcium imaging but also to other types of fluorescence imaging of OSNs.
Materials and Reagents
1.5 ml plastic tubes (Bio-bik, catalog number: CF-0150)
27 G needles for injection (Terumo, catalog number: NN-2719S)
1 ml syringe (Terumo, catalog number: 170215)
50 ml centrifuge tubes (Greiner, catalog number: 227261)
Teflon tube (Chiyoda, catalog number: TF-4-10)
KimWipes (Crecia, catalog number: S-200)
Cotton buds (Suzuran, catalog number: 102046)
Toothpicks (Yanagi, catalog number: J-613)
Disposable balance tray (Bio-bik, catalog number: AS-DS)
Cement solution (GC, Product name: Unifast II liquid 100 g)
Cement powder (GC, Product name: Unifast II powder A3 35 g)
Saline (Otsuka, catalog number: 3311401A7028)
Ketamine (Daiichi-Sankyo, catalog number: S9-019780)
Xylazine (Bayer, Product name: Rompun 2% w/v solution for injection 25 ml)
Vaseline (Wako, catalog number: 227-01211)
70% ethanol (Shinwa, catalog number: WK2-75)
Superglue (Sankyo, Product name: aron alpha A 0.5 g × 5)
Kwil-sil (WPI, catalog number: KWIK-SIL)
Valeraldehyde (Tokyo Chemical Industry, catalog number: V0001)
Mineral oil (Sigma, catalog number: M5310-500 ML)
Phosphate-buffered saline (PBS)
Mouse:
The OSN-specific GCaMP transgenic mouse line, OSN-GCaMP3 (OMP-tTA; TRE-GCaMP3 compound heterozygous bacterial artificial chromosome transgenic mice, 8-16 weeks of age) was used (Iwata et al., 2017; Inagaki et al., 2020). OMP-tTA (Accession# CDB0506T) and TRE-GCaMP3 (Accession# CDB0505T) are available from RIKEN (http://www2.clst.riken.jp/arg/index.html).
Note: Transgenic mouse lines expressing any indicators could be used, but sparsely and brightly labeled lines are preferred so that you can easily distinguish OSN responses. In OSN-GCaMP3 mice, GCaMP3 is expressed in 57.9% of the total OSNs (Iwata et al., 2017). A mouse line based on the Tet-system may be suitable for OE imaging in terms of labeling density and fluorescence intensity.
Equipment
Micropipette (Gilson, model: P200 and P1000)
Heating pad (Natsume, catalog number: KN-475-3-40)
Forceps (KFI, catalog number: 1-9749-32)
Fine forceps (Ideal-tek, catalog number: 91-2427)
Fine scissors (Mizuho, catalog number: 04-001-13)
Fluorescent stereomicroscope (Leica, model: M205 C)
Note: An epifluorescence microscope is useful for assessing the thickness of the skull above the OE (detailed below).
External light source for fluorescence excitation (Leica, model: EL6000)
Filter cube (Leica, model: GFP)
Head holder for surgery (Narishige, model: SG-4N)
Custom-made aluminum nose bar (Figure 1)
Note: We installed a custom-made nose bar to Narishige SG-4N (Figure 1A), as the original nose bar was too long and prevented surgical access to the OE. The size should be adjusted to your head holder and to make the skull over the OE accessible during surgery (Figure 1C). Any head holders can be used as long as the OE is accessible for surgery.
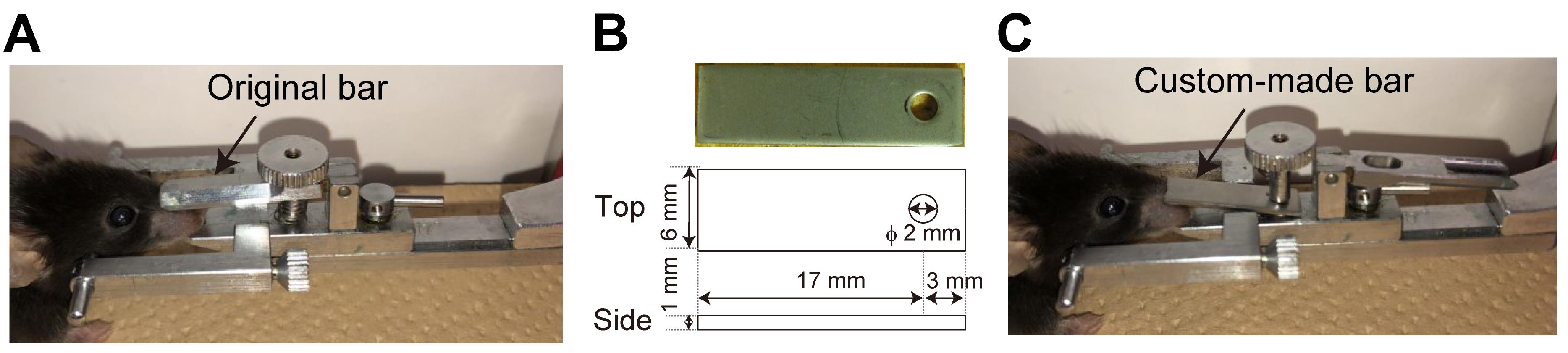
Figure 1. Installation of a custom-made nose bar to a head holder. A. An original nose bar of a head holder. B. The design of a custom-made nose bar. C. The custom-made nose bar is installed into SG-4N.Custom-made aluminum head bar (4 × 22 mm)
Note: The head bar was designed for a custom-built head holder as described in a previous study (Guo et al., 2014).
Custom-built head holder for imaging
Note: The head holder was built as described previously (Guo et al., 2014). Any head-holding system can be used for this protocol if the OE is accessible for in vivo imaging.
Φ1 mm drill tip (Meisinger, catalog number: ST1 HP010)
Dental drill (Leutor, model: LP-120)
Dust blower (UN, catalog number: UN-1321)
Two-photon microscope (Olympus, model: FV1000MPE)
Fluoview software (Olympus, model: FV10-ASW)
25× objective lens (Olympus, model: XLPLN25XWMP)
Custom-built olfactometer
Note: The design of the olfactometers has been described elsewhere (Slotnick and Restrepo, 2005; Burton et al., 2019). Briefly, the olfactometer consists of an air pump (AS ONE, catalog number: 1-7482-11), activated charcoal filter (Advantec, model: TCC-A1-S0C0 and 1TS-B), and flowmeters (Kofloc, model: RK-1250)]
Procedure
文章信息
版权信息
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Inagaki, S., Iwata, R. and Imai, T. (2021). In vivo Optical Access to Olfactory Sensory Neurons in the Mouse Olfactory Epithelium. Bio-protocol 11(12): e4055. DOI: 10.21769/BioProtoc.4055.
分类
神经科学 > 行为神经科学 > 嗅觉
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link